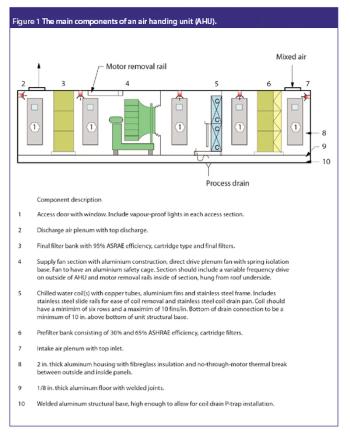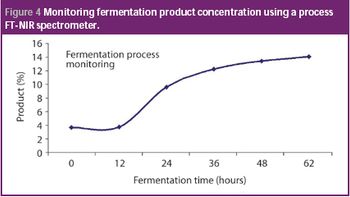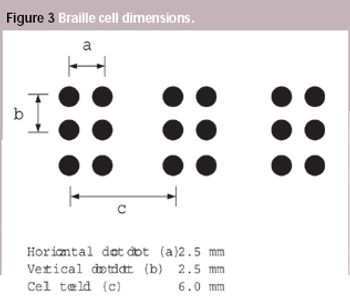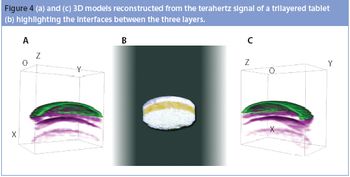
Pharmaceutical Technology Europe
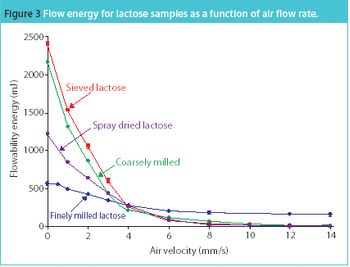
The trend towards developing pharmaceutical products and their manufacturing processes in tandem supports optimized production. Such developments rely on gathering process-relevant information at an early stage and being able to draw on past and current processing experience. Here, we discuss how powder rheometers can make a real difference in building a database of powder properties and removing subjectivity.