
Contract manufacturing organizations that are established in key markets can provide a competitive edge.

Contract manufacturing organizations that are established in key markets can provide a competitive edge.
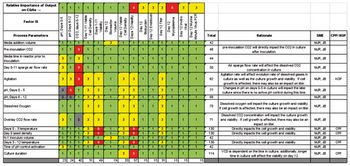
Traditional project decision-making is compared with a QbD approach.

Geographic location, customs and drug-development regulations knowledge, and transportation infrastructure were factors in Catalent Pharma Solutions' selection of Shanghai for its clinical-trials supply facility.

The relatively low success rates for bio/pharmaceutical compounds in clinical development have prompted many organizations to explore ways of reducing risk in clinical development.

Increased competition from CMOs in Asia means that Western CMOs need to understand fundamental changes in the market.
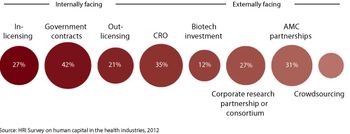
Pharmaceutical companies are adopting multiple choice partnership strategies to meet the challenges of today's R&D instead of being focused primarily on internal development.

A changing biopharmaceutical industry is going beyond typically outsourced activities and is using CMOs for more challenging processes. Review the top 10 outsourcing trends.

For a drug-development process that relies on outsourced services, special considerations are needed to ensure the proper transfer of technology and information from one phase to the next.

Jubilant HollisterStier