Particle size analysis
Pharmaceutical Technology Europe
An expert on diffraction systems offers insight on particle size specifications and analysis.
Do I need particle size specifications for my pharmaceutical product?
Specifications define the critical to quality factors to which a pharmaceutical product must conform to be acceptable for its intended application. Quality by Design highlights the importance of identifying and controlling all the parameters that can influence final product performance: ICH Topic Q6A identifies particle size as a potentially important variable.1 The specific need for a particle size specification is determined by assessing if and how this parameter affects performance.
For solid or suspension delivery systems, bioavailability is often directly related to particle size because it controls dissolution/solubility characteristics. Dissolution rate is directly proportional to particle surface area (Noyes-Whitney equation), so a finer particle size promotes faster drug dissolution. Particle size distribution is also relevant as a narrow distribution produces more uniform dissolution. Formulations with even a small number of relatively large particles may take some time to dissolve completely, but this may be the design intent.
For suspensions, stability is an important issue because if the active ingredient settles there is a greater chance of non-uniform delivery. Stokes' law relates settling velocity to the physical characteristics of the fluid and the size of particles in the suspension. The relationship here is a strong one: velocity correlates with the square of particle diameter. For suspension stability, a very low settling velocity is preferable and is more easily achieved with finer particles.
Perhaps less obviously, particle size may also affect a formulation's behaviour during processing and, ultimately, its content uniformity, which is critical. Take, for example, the widespread operation of direct compression tableting; particle size can influence segregation behaviour, the ease with which powder flows through the press and the compressibility of a formulation. In turn, these factors affect the consistency of tablet weight and composition, how the press operates and the mechanical properties of the finished product.
Figure 1 shows a near-infrared chemical image of a recalled tablet. The active ingredientrich domains (the red areas) are much larger in the right side of the tablet than in the left, demonstrating that composition is not uniform. The product was recalled in this case as the larger particle size may have caused some unit doses to fail potency specifications. Poor control of particle size clearly resulted in a substandard product.
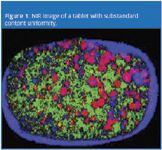
Figure 1
As analytical techniques become more sophisticated, researchers are increasingly able to show that particle shape, as well as size, can be influential. The tableting example illustrates this point as segregation, flow properties and compressibility are a function of both size and shape, not size alone. It is not uncommon to find particle size specifications supplemented with complementary shape criteria.
In summary, if any critical performance attributes depend on particle size or shape, a suitable specification is needed. If other variables can fully define performance, then none is necessary. In practice, particle size specifications are common within the industry for tablets, dry powder inhalers, suspensions and creams.
What issues should be considered when selecting a particle sizing technique/instrument?
Once the need for a particle size specification is established, it must be defined in the most relevant way. Here, an understanding of different particle size measurement techniques (operating principles and the reported variable) is essential. There are many ways of defining particle size, but only perfect spheres, which are rare in the manufacturing environment, can be described fully using a single number (a diameter). For particles with irregular shapes, there are multiple, equally valid measures of size including:
- equivalent volume diameter (diameter of a sphere with the same volume)
- circle equivalent diameter (diameter of a circle with the same projected area)
- longest dimension (arguably most relevant when particles are highly irregular — long and thin).
The ideal technique will report data that correlate closely with a given performance attribute. Measured parameter is one aspect of this, but the basis for the distribution is also important. With a number-based particle size distribution, the number of particles in each size range is reported; with a volume-based distribution, it is the volume, or for particles of constant density, the mass of material. In the case of pharmaceutical products, a mass or volumeweighted distribution is often the most relevant descriptor of the content of active ingredient as a function of particle size.2 Volumebased methods may be more sensitive to the presence of larger particles and aid the detection of the end point for unit processes, such as milling. However, number-based methods may improve detection of small amounts of fine particles, which may impact properties such as powder flowability and dissolution rate. Therefore, the choice of technique depends on the application.
More pragmatic issues include the speed of measurement and how easy it is to transfer the technique from the laboratory to the manufacturing floor. During the early stages of a development, longer analytical times may be acceptable, particularly if the results are information rich. For particle characterization, image analysis can be an excellent choice because it provides statistically relevant size and shape data as well as visual images, which in combination give detailed insight into the product. Measurement times are relatively long (approximately 15–20 min), but still much shorter than using manual microscopy.
In contrast, PAT must offer much more rapid analysis because the aim is measurement of a dynamic process. Where extremely fast analysis is required, other techniques become more valuable. Laser diffraction, for instance, provides a complete volumebased distribution in milliseconds. This makes it extremely productive for particle characterization in the laboratory and equally valuable for the realtime monitoring of processes, including spray events.
Whatever the analytical method, many of the issues regarding instrument choice are shared — ease of use, flexibility and reproducibility are all key. For laser diffraction, decades of development have streamlined analytical methods; the best instruments now offer fully automated operation driven by standard operating procedures (SOPs). The repeatability of such systems is exceptionally high. Advances in the core technology have also delivered improvements in fundamental performance, broadening measurement ranges. A single instrument can be used for many different samples, building greater value into the investment.
How do I develop and validate a methodology for particle size analysis?
If a particle size specification is required, then so too is an analytical method that will accurately reflect whether or not a material meets the specification. Measurement should be repeatable, reproducible and robust.
If a method is highly repeatable it means that running the same sample on the same instrument produces very similar results. Repeatability is determined by instrument performance and the state of dispersion of the sample. Typically, the aim is primary particle size measurement, in which case it is necessary to disperse any agglomerates/aggregates prior to analysis. Research has shown that inadequate dispersion is the greatest source of measurement error for particles with a diameter of 20 μm or less (Figure 2).3

Figure 2
Laser diffraction can be used for either wet or dry analysis. Wet sample dispersion involves the use of a suitable dispersant to separate the particles, followed by stabilization with admixtures or surfactants to prevent recombination of the particles during measurement. Vigorous stirring or ultrasound treatment promotes initial dispersion. With a dry system, the air pressure used to draw the sample into the measurement zone can be manipulated to control the degree of dispersion.
A wellengineered instrument will produce repeatable measurements provided dispersing conditions are appropriate and well defined, and the concentration of particles in the measurement zone is suitable. However, reproducible measurement is a more exacting requirement. A highly reproducible procedure gives accurate and precise results for different samples, so sampling methodology is a contributory factor. The US and European pharmacopoeias specify a target reproducibility of 10% for the volume median diameter (Dv(50)) for particles >10 μm in diameter and 20% for those that are smaller.4,5 This figure takes into account all the errors associated with the entire analytical process.
Many analysts underestimate errors that arise from sampling. Laboratory analysis involves measuring a small amount of material to determine the properties of a much larger bulk. Ensuring the sample is truly representative is essential. It is estimated that for systems containing particles >75 μm in diameter, sampling is the greatest source of error.3 As with dispersion, it is sensible during method development to investigate the impact of sampling technique on size measurement and to closely define a procedure.
Comparison of different measurement techniques can provide valuable insight for method development; for example, image analysis provides size and shape data that verify effective dispersion. With dispersion, the aim is to apply sufficient energy to disperse the particles without breaking them up. Image analysis is a relatively straightforward way of determining whether or not this aim has been achieved. Comparing image analysis and laser diffraction data is also a good way of confirming that the optical properties being used for diffraction analysis are correct.
Validation is the final step in the development of a particle size specification and an associated measurement method. FDA guidance states that, for particle sizing, "methods validation usually involves an evaluation of intermediate precision and robustness".6 Intermediate precision is related to both repeatability and reproducibility, and is tested by measuring the same sample, on a different day, using a different instrument. Robustness is assessed by evaluating the impact of small changes to the methodology on the results.
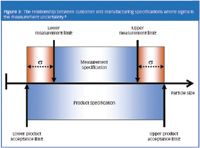
Figure 3
FDA guidance also states that "assurance should be provided that the data generated are reproducible and control the product's quality".6 Effective method development, particularly when implemented through SOPs, will give reproducible analysis. For the particle specification to control product quality, it essential to have effective definition that considers the variability associated with measurement. Understanding the particle size range that gives acceptable performance boundaries for a product enables the specification to be set. The specification will be narrower than the true window of acceptability by an amount that depends on the variability of the analytical method (Figure 3). If the product meets this specification then it will deliver the required performance.
Paul Kippax is Product Manager Diffraction Systems at Malvern Instruments Ltd (UK).
References
1. ICH Topic Q6A — Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances, December 2000. www.ich.org
2. D.J. Burgess et al., AAPS J., 6(3), article 20 (2004).
3. H. Merkus "Quality Assurance in Particle Size Measurement" from Improving Standards in Particle Size Distribution Measurement, Engineering Research Centre for Particle Science and Technology (Gainsville, FL, USA, February 1997).
4. General Chapter <429> (2008), "Light Diffraction Measurements of Particle Size," United States Pharmacopeia. www.usp.org
5. European Pharmacopoeia, Chapter 2.9.31, Laser Diffraction Measurement of Particle Size, Supplement 5.6 (2006), p 4429. www.edqm.eu
6. FDA Draft Guidance — Analytical Procedures and Methods Validation, August 2000. www.fda.gov
7. D.J. Wheeler, How to Establish Manufacturing Specifications (2003). www.spcpress.com
Drug Solutions Podcast: Novel Drug Delivery Approaches: Refining AAV Vector Deliveries
May 30th 2025In this podcast episode, we discuss novel approaches to drug delivery, specifically regarding adeno-associated virus (AAV) vectors, as viewed by two industry experts who recently exhibited at the annual ASGCT meeting.
Prokaryotics Licenses Gram-Negative Antibiotic Potentiator from Northern Antibiotics
June 26th 2025Prokaryotics will gain worldwide rights to develop, manufacture, and commercialize NAB741, a non-bioactive polymyxin designed to increase permeability of the outer membrane of Gram-negative bacteria.
Transformations in Drug Development for Cell and Gene Therapies
March 28th 2025As a recognized leader in immunophenotyping for clinical trials, Kevin Lang from PPD discusses how spectral flow cytometry is transforming drug development, particularly in cell and gene therapies like CAR-T. He also dives into his award-winning research, including his 2024 WRIB Poster Award-winning work, and his insights from presenting at AAPS PharmSci360.
