
Equipment and Processing Report
In a 2004 guidance, FDA says that the "use of redundant sterilizing filters should be considered in many cases." But not all manufacturers agree on what redundant filtration is.

Equipment and Processing Report
In a 2004 guidance, FDA says that the "use of redundant sterilizing filters should be considered in many cases." But not all manufacturers agree on what redundant filtration is.

Equipment and Processing Report
PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2011 edition from Lechler and National Bulk Equipment.
Equipment and Processing Report
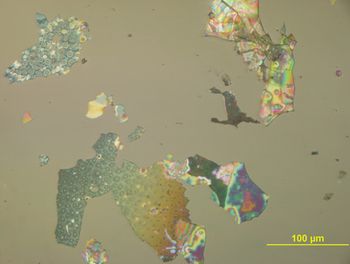
Glass flaking or delamination can result in a failed quality-assurance test, thus bringing production to a halt and causing substantial revenue loss. If glass delamination remains undiscovered, it can pose a serious contamination risk to the drug product and a potential health risk to the public.

Equipment and Processing Report
In our manufacturing process, we are running into issues with our vial stoppers clumping in the feeder bowl. How can we ensure that the stoppers go through smoothly without clumping up?