
Pharmaceutical Technology Europe
In view of the nature of its complexity, it might be desirable to apply FDA's process analytical technology to lyophilization.

Pharmaceutical Technology Europe
In view of the nature of its complexity, it might be desirable to apply FDA's process analytical technology to lyophilization.

Pharmaceutical Technology Europe
UK science has an outstanding record and we remain strong internationally in terms of achievement, productivity and efficiency.
Pharmaceutical Technology Europe
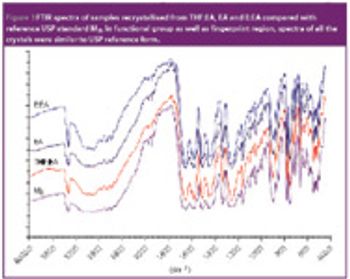
Mefenamic acid has variable bioavailability and tabletting issues because of its hydrophobic nature and poor material characteristics. Recrystallization of mefenamic acid was performed from three different solvent–solvent mixtures under differing conditions. The crystals obtained were screened for the existence of new crystal properties or polymorphic forms, then characterized further.
Pharmaceutical Technology Europe
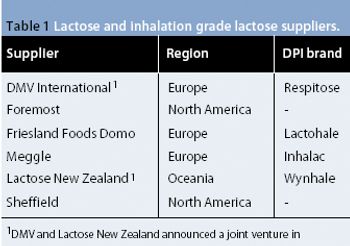
The presence of very low levels of residues (including solvents) in excipients is becoming an important issue for users, and the presence of very low levels of ?non-lactose? species in DPI lactose may pose challenges to suppliers and users.

Pharmaceutical Technology Europe
Developed in the 1950s as a means to survive and compete against the giants of the automotive sector, lean manufacturing helped Toyota evolve from a small-volume producer (with little capital) to become a high-volume manufacturer in a process-rich environment. Toyota achieved this by using developments such as total production maintenance (TPM), just-in-time (JIT), Kanban, value stream mapping and Kaizen events.1 A summary of some of the lean terminology is shown in Table 1.

Pharmaceutical Technology Europe
It will remain to be seen whether pharmaceutical companies will commit lock, stock and barrel to being green.