
Solid Dosage and Excipients

Solid Dosage and Excipients

The author reviews significant changes to GMP for excipients in the forthcoming American National Standard, including a risk-based approach to excipient manufacture, why new requirements were proposed, and their potential impact to excipient manufacturers.

The article examines some recent developments for this process step and for continuous manufacturing overall.

This article provides guidance for industry on how to comply with the pending American National Standard on excipient GMP, with a focus on risk assessment.
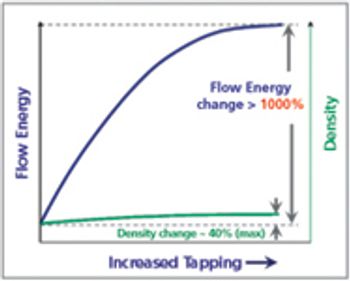
This article considers the different conditions to which the powder is subjected in the tableting process, and discusses which powder properties should be measured to accurately reflect likely powder behavior in the process.
Pharmaceutical companies, equipment providers, contract-service providers, and excipient manufacturers apply various approaches for improving solubility. The article examines some recent developments.

Taste-masking is an important consideration to ensure patient compliance.

Experts in solid dosage discuss the formulation and manufacture of multilayer tablets.

FDA answers key questions about the October 2011 guidance on using physical–chemical identifiers in solid oral dosage products to help prevent and avoid counterfeiting.