
The author explains the planning, equipment, and facility design requried for manufacturing HPAPIs and specialized requirements for handling these compounds.

The author explains the planning, equipment, and facility design requried for manufacturing HPAPIs and specialized requirements for handling these compounds.

The outlook is fairly optimistic as contract manufacturing organizations (CMOs) gather at CPhI Worldwide in Frankfurt. CMOs are expanding capacity for small-molecules, biologics, and finished-product manufacturing.
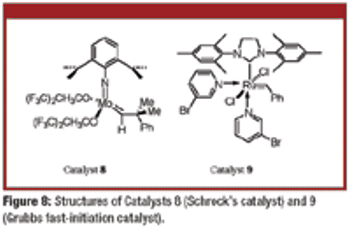
The authors describe the Piers' catalysts and detail latest progress in olefin-metathesis catalyst technology.
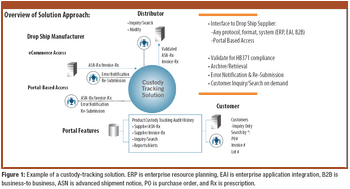
EPedigree, track-and-trace technologies, and other tools for optimizing supply-chain management are of increasing importance to the pharmaceutical industry. The author examines the current regulatory and legislative framework for ePedigree for finished drug products as well as proposals to require electronic statements for pharmaceutical ingredients.
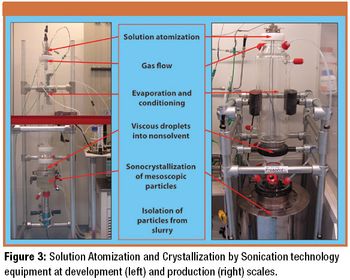
The authors discuss advanced sonocrystallization particle-engineering techniques for manufacturing mesoscopic particles.

Recombinant microbial whole-cell biocatalysis is a valuable approach for producing enantiomerically pure intermediates. The authors examine several groups of enzymes using this approach: dehydrogenases, hydantoinases, and acylases.

The authors describe the critical aspects of an ideal fermentation services provider.