
The extensible markup language (XML) format facilitates compliance with FDA's new requirements for prescription drug labeling submissions, improves patient safety, and enhances manufacturing sponsor efficiencies.

The extensible markup language (XML) format facilitates compliance with FDA's new requirements for prescription drug labeling submissions, improves patient safety, and enhances manufacturing sponsor efficiencies.
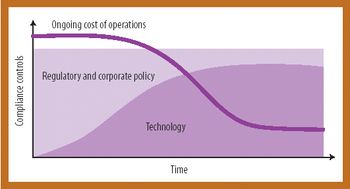
Information technology can streamline compliance and increase operational efficiency and quality.
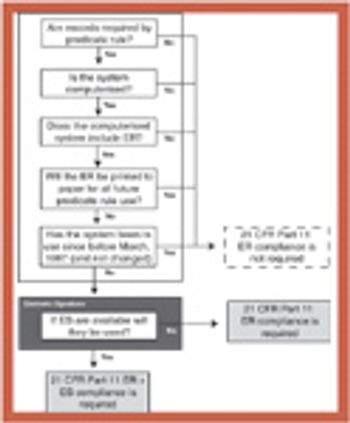
Coordinating validation efforts throughout an organization requires an accurate and timely overview and a validation master plan (VMP).

To implement process analytical technology systems into the current information technology landscape, manufacturers will need to adopt continuously available systems and infrastructures.
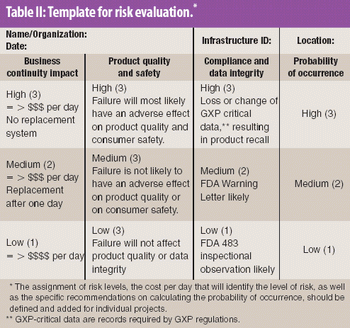
Risk analysis and evaluation of software and computer systems is a good tool to optimize validation costs by focusing on systems with high impact on both the business and compliance.