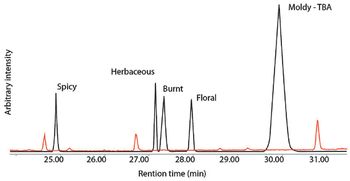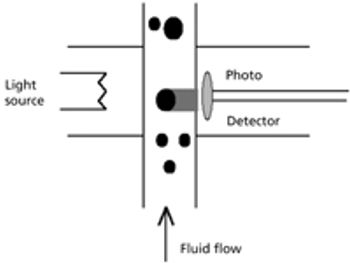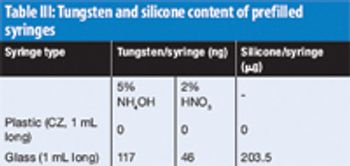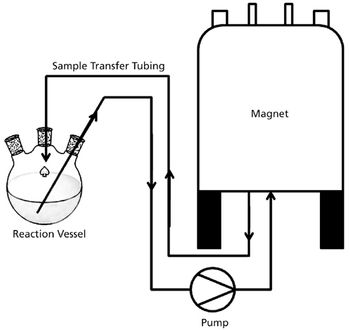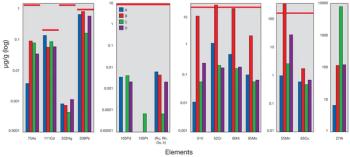
The US Pharmacopeia (USP) proposes to lower the maximum permissible limits of trace elements in pharmaceuticals and recommends that impurities be measured through automated instrumentation-based methods. The proposed regulations specify inductively coupled plasma–mass spectrometry (ICP–MS) and inductively coupled plasma–optical emission spectrometry (ICP–OES) as the techniques of choice. This article discusses the benefits of ICP–MS and ICP–OES for the accurate detection of trace elements in pharmaceutical products, in compliance with the proposed USP chapters.