- Pharmaceutical Technology-12-02-2017
- Volume 41
- Issue 12
Formulating a Recipe for Bio/Pharma Career Success
Attracting and retaining qualified bio/pharma experts demands a mix of recognition, rewards, and opportunities.
An efficient, effective drug development and manufacturing operation is crafted by talented, experienced pharmaceutical scientists and engineers. Synthesizing the recipe for success can be daunting in the current business environment.
The US unemployment rate, at 4.1%, is at the lowest level in 16 years. More complex molecules demand more sophisticated formulation methods. Advanced technology for pharmaceutical manufacturing will increase the required skill sets of bio/pharma workers. The industry also faces gaps in know-how and expertise as “baby boomer” employees head to retirement.
These factors suggest a challenge for bio/pharma employers and a positive employment picture for bio/pharma employees seeking to start or advance careers in the bio/pharmaceutical industry. Mix in financial pressures from shareholders and payers, pressure to reduce costs, globalization, and generic-drug competition, however, and the final result may be conflict between employer priorities and employee career paths.
Respondents to Pharmaceutical Technology’s annual employment survey (1) shared opinions about the current employment environment, expressed reservations about compensation and workloads, and indicated that better career opportunities were a top priority.
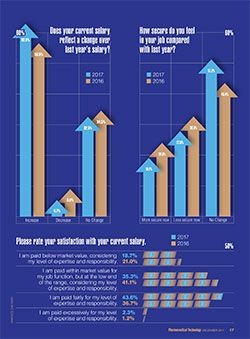
While nearly 40% of the respondents reported that business increased compared to 2016, fewer respondents felt “more secure” in their positions in 2017 (19.1%) versus 24% in 2016 (2); however, fewer respondents said they felt less secure in 2017 (27.8%) compared with 32.6% in 2016 (
).
Opinions about the job market varied. While 44.3% said the job market was moderately competitive, one-quarter said the market for jobs was competitive; there are more qualified candidates than open jobs. The remaining 30% said there are few qualified applicants for open positions and employers must compete for qualified candidates.
Survey respondent profile
More than 480 bio/pharma professionals from around the world responded to the survey, which was fielded in September and October 2017. Respondents primarily were full-time, permanent employees (87.3% of respondents) at innovator bio/pharmaceutical companies (33.8%), generic-drug manufacturing companies (11.6%), contract research and manufacturing organizations (15.8%), and consulting firms (6.4%).
The represented companies develop or manufacture both small- and large-molecule drugs, vaccines, and cell therapy or regenerative medicines at privately held companies (40.4%), publicly traded companies (40.2%), and non-profit/academic/government groups (14.2%).
Respondents reported a range of job responsibilities from R&D, to manufacturing, to quality control/assurance. More than 42% work for companies with more than 5000 employees; more than 34% work for companies with fewer than 500 employees.
Compared with previous years, the respondents reported more experience working in the bio/pharma industry; 24.6% had fewer than 10 years of experience, 28.8% had 10-20 years, 37.4% had 20-35 years of experience, and 9.2% have worked in the industry for more than 35 years. About half of the respondents said they worked outside the bio/pharma industry for up to five years.
More work, less play …
Workloads increase slightly in 2017, with 60.9% reporting increases compared with 59.4% in 2016. More than one-third of the respondents (36.5%) say they worked more hours in 2017 than two years ago, similar to responses in previous years. While 56.3% of the respondents said they were contracted to work approximately 40 hours per week, only 21.6% reported working 40 hours. Nearly one-third (31.4%) said they were contracted for more than 40 hours per week; however, more than 70% of the respondents said they work 40 or more hours per week.
More than 42% of US-based employees reported two to three weeks of vacation time; 37.1% reported four to six weeks. In comparison, 87% of the European-based respondents reported four or more weeks of paid vacation. Of all respondents, 27.3% reported that they used their full allotment of vacation, personal, and sick time. A similar percentage (22.5%) said they used less than half of the available time off. European-based respondents (70%) were more likely to use at least three-quarters of their paid time off compared to US-based respondents (57.4%).
But more pay
Nearly two-thirds of the respondents (62.9%) reported pay increases in 2017, up from 56.5% in 2016, the highest percentage reporting increases since 2014. Satisfaction with salary levels improved compared to the past two years; however, more than half (54%) were dissatisfied with their compensation. More than 35% of the respondents said they were paid at the low end of the salary range for their job function for their expertise and responsibility; 19% said they were paid below market value (
).
Benefits are also a key element of employee satisfaction. While more than one-third (36.2%) of the respondents reported an increase in the cost of health insurance, only 8% noted an improvement in coverage. Retirement benefits were unchanged; less than 14% reported an increase in bonuses.
In for the long term?
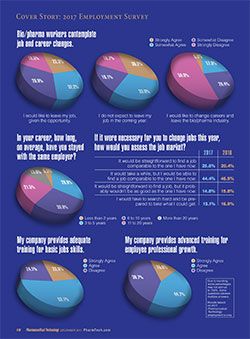
While three-quarters of the respondents have more than 10 years of experience in bio/pharma, 28.9% stayed with the same employer--on average--for three to five years, a decline from 34.1% in 2016. More than 30% of the 2017 respondents said they stayed with an employer for an average of 6-10 years; 35.3% stayed with the same employer for more than 10 years (
).
In addition to salary and benefits, employee recognition can play a role in job satisfaction. Most respondents said their work is fully valued by their employer (32.2% strongly agreed; 46.2% agreed). More than three-quarters of respondents said their skills and training are used to the fullest level. Only 56.8% see opportunity for career advancement in their current position; 68.4% reported opportunities for professional advancement at their current companies.
Compared to previous surveys, fewer respondents to the 2017 survey identified a single factor as motivation to work or a sole reason to change jobs. Challenging projects (33.2%), intellectual stimulation (33.2%), a good work/life balance (26.6%), and a high salary (26.3%) were the top factors respondents cited as “the main reason I come to work.” Work/life balance (33.2%), professional advancement (30.6%), and salary (28.3%) were the top factors for changing jobs. Overall, respondents were tolerant of negative workplace activity; only a small fraction cited single factors that would cause them to quit their jobs; issues with management (14.9%), low pay (12.3%), discrimination (11.5%), and negative attitudes (11.4%) were most frequently cited.
Seeking better opportunities
More than half of the 2017 respondents (54.6%) “agreed somewhat” or “agreed strongly” that they would “like to leave their job, given the opportunity,” down slightly from the past two years. More than one-third said they were “likely to leave my job voluntarily in the coming year,” and 13.5% expected to leave their job involuntarily. In a somewhat concerning result, nearly 23% said they would like to change careers and leave the bio/pharma industry (
).
While the desire to change positions is strong, the number of people actually changing jobs is significantly less, but growing. In 2016, 17.2% of respondents reported a voluntary job change; in 2017, that number jumped to 21.7%.
Confidence levels of those seeking new bio/pharma positions continued to rise in 2017; 25.8% said it would be straightforward to find a comparable new job; 44.4% said it may take a while, but they would be able to find a comparable position. The percentage of less-optimistic job seekers declined slightly, 14.8% said it would be straightforward to find a job, but the new job probably would not be as good as the current position; 15.1% anticipated a difficult search and they would have taken whatever position was available (
).
References
1. 2017 Pharmaceutical Technology/Pharmaceutical Technology Europe Employment Survey, Pharmaceutical Technology, 2017.
2. 2016 Pharmaceutical Technology/Pharmaceutical Technology Europe Employment Survey, Pharmaceutical Technology, 2017.
Article Details
Pharmaceutical Technology
Vol. 41, No. 12
December 2017
Page: 16–19
Citation
When referring to this article, please cite it as R. Peters, “Formulating a Recipe for Bio/Pharma Career Success," Pharmaceutical Technology 41 (12) 2017.
Articles in this issue
about 8 years ago
Disputes Over Manufacturing Waiver and Other SPC Exemptionsabout 8 years ago
Opportunities with Softgelsabout 8 years ago
What’s in Your SOP?about 8 years ago
Transformative Medicines Challenge FDA and Manufacturersabout 8 years ago
Pharma Outsourcing: A Year in Reviewabout 8 years ago
Outsourcing: A Year in Reviewabout 8 years ago
Designing Sustainable Pharma Facilitiesabout 8 years ago
Evaluating Surface Cleanliness Using a Risk-Based Approachabout 8 years ago
Wanted: A Highly Skilled Bio/Pharma WorkforceNewsletter
Get the essential updates shaping the future of pharma manufacturing and compliance—subscribe today to Pharmaceutical Technology and never miss a breakthrough.