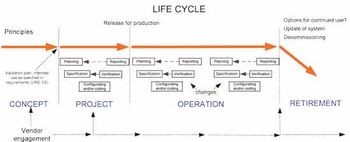
Non-compliance issues show that users find dealing with computer systems challenging.
Henrik Johanning is CEO and specialist, Genau & Co, Denmark. He is also a member of Pharmaceutical Technology Europe’s Editorial Advisory Board.

Non-compliance issues show that users find dealing with computer systems challenging.

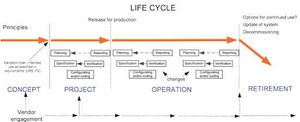
Though the pharma industry has improved its change management processes, there are still opportunities for improvement.
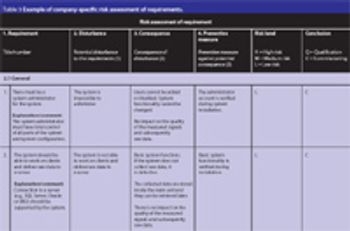
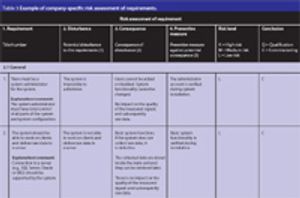
It is becoming evident that quality risk management within regulated, life sciences environments is a valuable component of an effective quality management system (QMS). A QMS provides a proactive and systematic means to identify, analyse, evaluate and control potential process and product quality issues during development, manufacturing, distribution and marketing throughout the entire product life cycle.
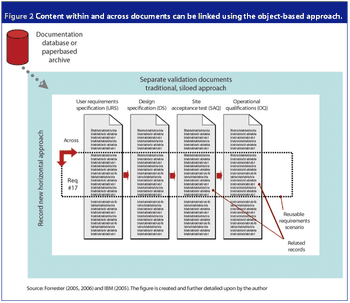
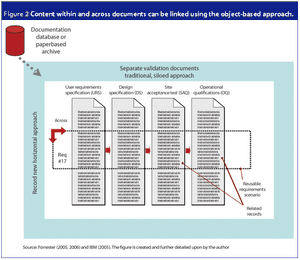
An electronic object-based approach towards validation is forecasted to be the upcoming validation paradigm supported by reliable web-based technology, organizational focus on risk management and overall enterprise effectiveness.
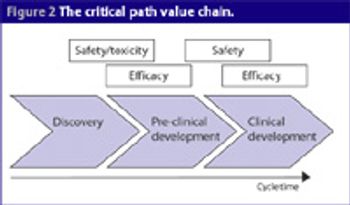
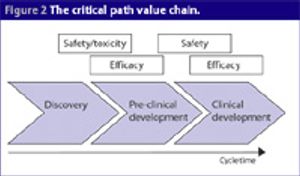
One of the biggest barriers research and academic institutions face is the ability to get discoveries made in the lab into clinical testing. Because only small amounts of drugs are used in these early studies, they represent fewer potential risks for people in these trials.

Published: May 1st 2010 | Updated:
Published: October 1st 2007 | Updated:
Published: December 1st 2007 | Updated:
Published: July 1st 2006 | Updated:
Published: February 2nd 2014 | Updated: