
The authors used common solvents to develop an initial solvent-screening method for laboratory-scale research to determine the solubility, polymorphism, and crystal properties of various active ingredients.

The authors used common solvents to develop an initial solvent-screening method for laboratory-scale research to determine the solubility, polymorphism, and crystal properties of various active ingredients.

In Part I of this article, which appeard in the March 2010 issue, the authors describe their approach for constructing form spaces for carbamazepine, cimetidine, and phenylbutazone by initial solvent screening to evaluate the feasibility of spherical crystallization. Part II of this article discusses their findings.
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.
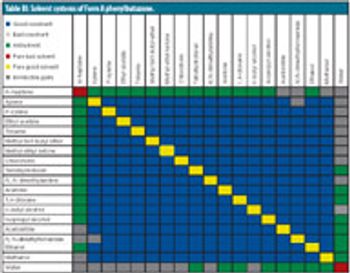
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.
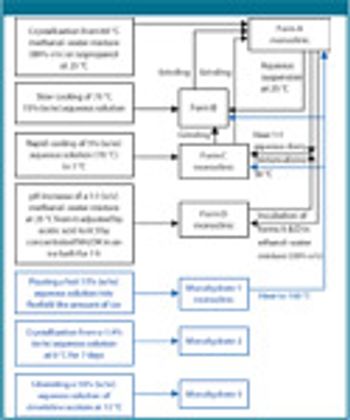
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.
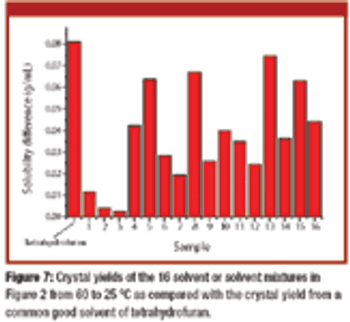
The authors propose extending initial solvent screening for a single-solvent system to the cocktail solvent screening of binary and ternary solvent mixtures.
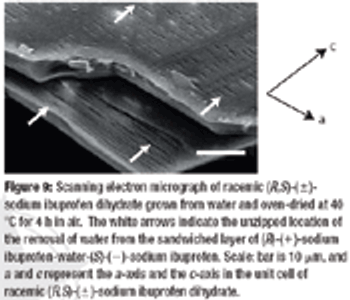
The racemic compound (R, S)-(±)-ibuprofen is a popular and well understood active pharmaceutical ingredient, but it has several disadvantageous formulation properties such as poor solubility, low melting point, and potential esterification with excipients containing an hydroxyl group. The authors investigate the use of an (R, S)-(±)-ibuprofen salt to evaluate these problems using various analytical methods to determine the polymorphism, crystallinity, and drying scheme.
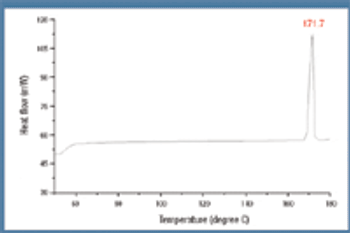
Solubility, polymorphism, crystallinity, and crystal habit of acetaminophen and racemic (+/-)-ibuprofen were determined by initial screening of 23 solvents for scale-up. Solubility curves were constructed, and solubility at 25 degrees Celsius was plotted against the dielectric constants of various solvent as a fingerprint for solute identification. The total "form space" for acetaminophen and racemic (+/-)-ibuprofen were calculated to be 222 and 257, respectively. Various crystal habits and sizes for ibuprofen and acetaminophen were observed.
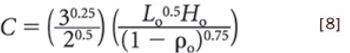
Quantitative data from the literature show strong relationships among average particle size, powder densification, tensile strength, and hardness.
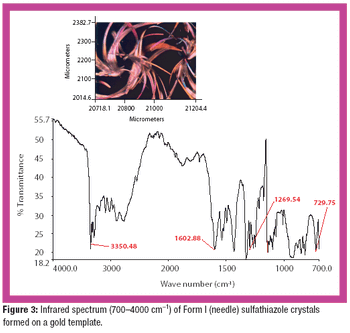
Polymorph farming on a chip is a promising strategy for rapid polymorph discovery by transforming crystallization?crystal isolation?characterization into one time-efficient step.

The authors describe the particle?surface frictional forces involved in the operation of various dryers and discuss the affect on the degree of particle attrition.

A rapid screening approach is shown to accelerate sample preparation and characterization methods in solid dispersion technology by integrating spin casting on a silicon chip with optical microscopy.
Published: March 2nd 2010 | Updated:

Published: August 2nd 2010 | Updated:

Published: April 2nd 2010 | Updated:

Published: June 2nd 2009 | Updated:
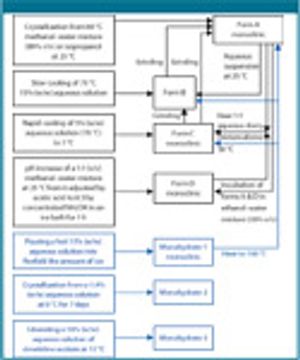
Published: May 2nd 2009 | Updated:
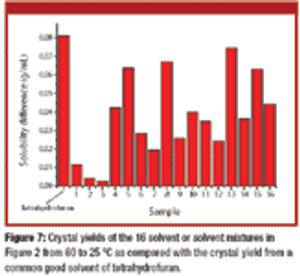
Published: January 2nd 2008 | Updated: