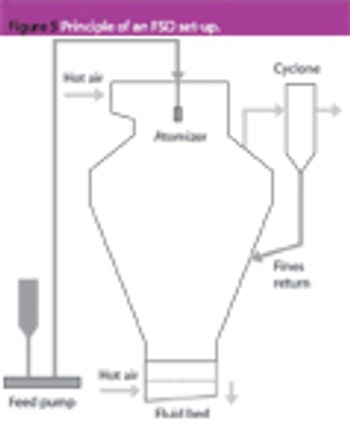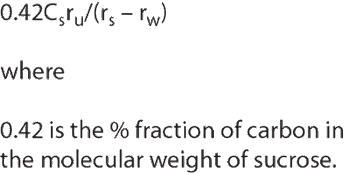
Pharmaceutical Technology Europe
The implementation of a total organic carbon (TOC) method into the United States Pharmacopeia (USP) has its origins in the early 1990s, when the Water Quality Committee (WQC) of the Pharmaceutical Manufacturers Association (PMA, later renamed PhRMA) debated improvements in the testing of purified water (PW) and water-for-injection (WFI). The resulting inclusion of modern analytical techniques replaced much older methods - some of which had been listed in the USP for more than 150 years. Finally, two new regulations were put in place: Chapter for conductivity, which replaced a series of individual ion tests; and Chapter which replaced the oxidizable substances test with a TOC method.