
AstraZeneca's purchase of Cambridge Antibody Technology and Merck's acquisitions of GlycoFi and Abmaxis are the latest efforts by pharmaceutical majors to build critical mass in biologics capabilities.

AstraZeneca's purchase of Cambridge Antibody Technology and Merck's acquisitions of GlycoFi and Abmaxis are the latest efforts by pharmaceutical majors to build critical mass in biologics capabilities.

Yet again, FDA's ability to regulate drugs is under fire. At the core of this latest round of scrutiny is whether the agency has the resources to properly control the safety of new nanotech-based drug products.
The correlation between swab assay results and visible-residue limits (VRLs) for cleaning validation was examined. Previously completed validation studies were reviewed to compare swab results with recently determined VRLs. A current cleaning validation study evaluated both swab testing and VRL. Unexpected swab results led to an investigation, which showed the value of establishing the VRL in conjunction with swab recoveries for cleaning validation programs.

Mid-size regional players have little chance for success in an industry increasingly dominated by large global players.
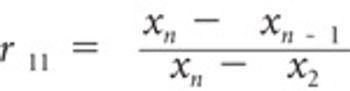
Outliers may provide useful information about the development and manufacturing process. Analysts use various statistical methods to evaluate outliers and to reduce their impact on the analysis. This article describes some of the more commonly used identification methods.

Here's to all the difficult, out-of-touch, and irresponsible coworkers that make our workplace interesting.
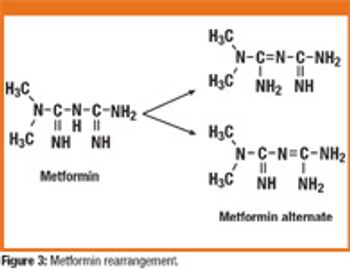
Optimizing formulation and API synthesis is critical for product success. Technology providers advance chemocatalysis for olefin metathesis and asymmetric reactions in API synthesis. And pharmaceutical majors share insights in formulation development.

FDA conducted 163 inspections of foreign API manufacturers in 2005.

FDA must monitor a growing number of facilities and complex drugs despite "resource challenges."

The reason for reading the report is to inoculate ourselves against error.

Branded pharmaceutical companies have long been immune to the cost pressures faced in other industries. With robust research and development pipelines, long periods of patent protection to ensure continued high margins, and a regulatory environment that has virtually eliminated the threat of overseas competition, pharmaceutical companies have traditionally considered spend management an afterthought. Recent changes in the pharmaceutical industry, however, have begun to erode Big Pharma's once-imperious position.
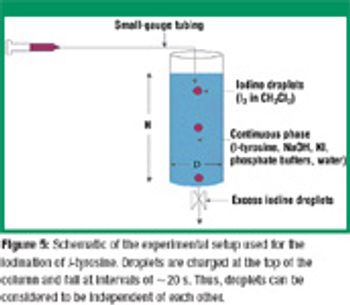
Understanding the impact of reactive mass transfer and local flow in multiphase systems is crucial for maximizing reaction selectivity and minimizing the formation of byproducts. The authors study the influence of mixing on fast liquid–liquid reactions. The iodination of L-tyrosine was used to demonstrate the relationship of droplet size and shape on reaction selectivity and to verify computational predictions. By understanding that droplet dynamics affect the yield and selectivity of fast reactions, the formation of byproducts can be minimized by optimizing operating parameters.