
This year's fall conference season clearly shows that the new, systematic approach to process planning and product quality is permeating the industry's thinking—if not yet its daily practice.

This year's fall conference season clearly shows that the new, systematic approach to process planning and product quality is permeating the industry's thinking—if not yet its daily practice.
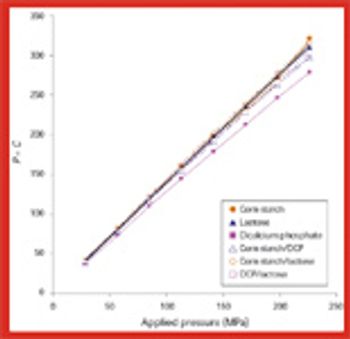
The survival of Bacillus subtilis spores in dicalcium phosphate, lactose, and corn starch and in their binary mixtures depends on the compressional properties of these materials and on parameters involved during the tableting process, including compression speed.

To qualify and validate a pharmaceutical manufacturing facility, one must carefully review the facility design for compliance with good manufacturing practices and manage project scope definition, labor and cost estimating, and master-plan development.These activities, properly implemented, help deliver a validated facility on schedule, at the estimated cost, and with expected quality.

A new oral dosage product was designed as "encapsulated tablets." In production, the drug product was pressed into tablets, which were then fed into a revolving capsule-filling table.

From politics to paychecks and downsizings to deadlines-what it's like to work for one of the world's largest industries.

If judges and juries lack the scientific knowledge to decide drugsafety cases, how can we protect both companies and patients?

CDER is reorganizing staffs and developing new policies to encourage industry adoption of quality production systems.

Roche (Basel, Switzerland, www.roche.com) has put a temporary halt on the distribution of the antiviral treatment, "Tamiflu" (oseltamivir phosphate), a neuraminidase inhibitor, to the United States in an effort to deter companies from stockpiling the antiviral for employee use, according to an article in the Oct. 27 edition of The New York Times.

First, a confession. Yes, the four sleep-deprived editors hunched behind glowing laptops in the Opryland Resort's Cyber Café last month were indeed from this publication. Normally, our mumbled conversations about punctuation and grammar take place before 11:00 pm—and under slightly lower systemic levels of caffeine, sugar, and sushi.
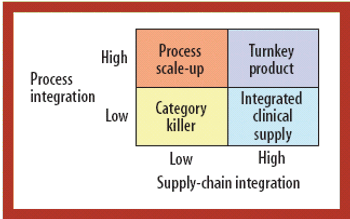
CROs and CMOs are pursuing new strategies to win a larger share of the burgeoning early development pipeline.