
Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

In this article, the authors look at the limitations of the validation for a single-use shipping system and provide a perspective on what shipping validation means.
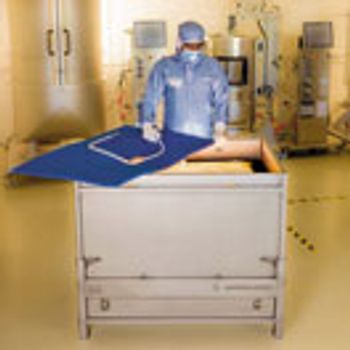
The authors provide their perspectives on shipping validation.
Published: March 1st 2016 | Updated:

Published: May 15th 2016 | Updated:

Published: May 2nd 2018 | Updated: