
Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.
Jean-Marc Cappia is vice-president of marketing for fluid management technology at Sartorius Stedim Biotech FMT, Aubagne, France.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

The authors describe the development and validation of a highly sensitive point-of-use pressure decay test.
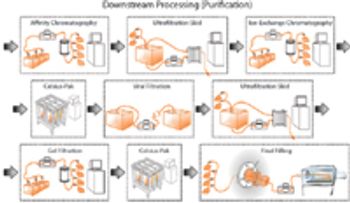
The authors discuss current and future disposable technologies and outline the validation and qualification steps that would be required for a possible disposable process stream.

Steaming-in-place (SIP) is a widely adopted method for the in-line sterilization of processing equipment. The main advantage of SIP relies on manipulation reduction and aseptic connections that might compromise the integrity of the downstream equipment.
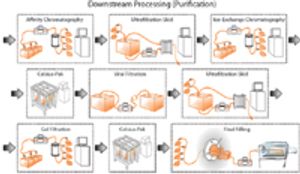
Published: May 1st 2009 | Updated:

Published: October 1st 2004 | Updated:

Published: January 2nd 2017 | Updated:

Published: May 2nd 2018 | Updated: