Pharmaceutical Technology Europe

Pharmaceutical Technology Europe

Pharmaceutical Technology Europe
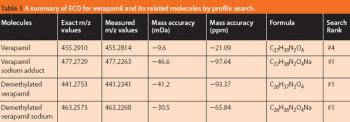
The Active Pharmaceutical Ingredients Committee (APIC) - a sector group of Conseil European des Federations de l'Industrie Chimique (CEFIC) - first voiced the need for EU GMP API legislation in 1993 to help ensure the safety of medicines. In 2000, the International Conference on Harmonisation (ICH) finalized the harmonized API GMP Guideline Q7, which became legal in the US and Japan in 2001. The EU adopted a directive in March 2004 that includes the requirement for APIs in medicines for the EU market to comply with ICH/Q7A. Member States are transposing the directive into their national law: about half of them have completed this process, seven more are well on their way to completion, while seven others are still in earlier stages of adoption.
Pharmaceutical Technology Europe
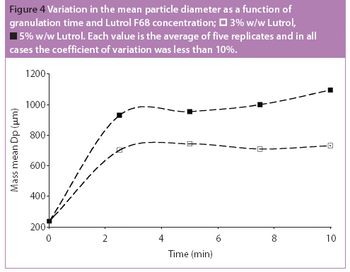
Fluidized hot melt granulation (FHMG) is an emerging technique combining the advantages of both dry and wet granulation methods, and represents an innovative continuous granulation process capable of mixing and agglomerating excipients and active pharmaceutical ingredients (APIs) to produce uniform blends of particles suitable for use in the manufacture of pharmaceutically elegant solid dosage forms.
Pharmaceutical Technology Europe
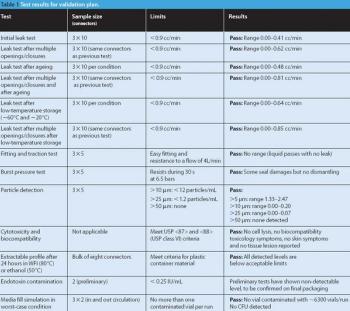
Sterile liquids are frequently transferred during the processing of sterile liquid drugs such as injectables or ophthalmic drops. Several types of transfer can be performed, each requiring a validated method to ensure the desired sterility-assurance levels are achieved.

Pharmaceutical Technology Europe
It has been a long time coming, but stakeholders in the US are now seriously debating a route to market for cheaper copies of biopharmaceutical drugs. The European Agency for the Evaluation of Medicinal Products (EMEA) has led the way on this issue by publishing clear guidelines on what companies must do to get their versions of drugs such as erythropoietin (EPO), an advanced treatment for anæmia, and similar products approved.
Pharmaceutical Technology Europe
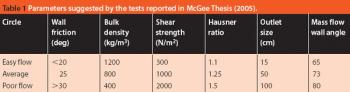
Predicting the flow characteristics of powders during manufacture is especially important for the pharmaceutical engineer. Getting the powder flow wrong can be highly disruptive to plant performance and productivity, particularly where equipment has to be taken off-line and stripped down for cleaning out blockages. The flow behaviour of the individual ingredients may be well known, but as these are blended and reacted their flow properties can change.

Pharmaceutical Technology Europe
The financial return to industry on its investment in academia through licensing and funding of basic research can be similar to that in its own research programmes. A report from the Royal Pharmaceutical Society, UK Drug Delivery Research: The Way Forward in the New Millennium, emphasized the importance of drug delivery research in improving patient outcomes and the opportunities for industry and academia to further explore this area, particularly for newly discovered macromolecular drugs. Unsurprisingly, therefore, industry has become increasingly interested in investing in research performed in academic settings as a complement to its own research. Government is currently exhorting universities to transform their traditional role to institutions that can drive economic development and innovation.