
How to avoid invisible and airborne contamination.

How to avoid invisible and airborne contamination.

Contamination is almost always related to human error and there is a clear drive to reduce human implications in aseptic operations. This can be achieved in multiple ways.

Closed vial technology has been designed to address the challenges - potential contamination, counterfeiting and process complexity - associated with the aseptic filling of injectable drugs.
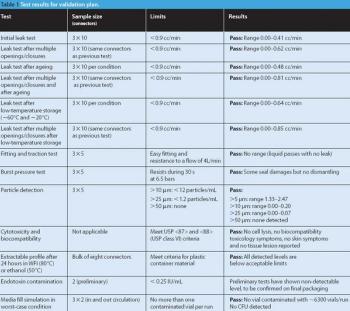
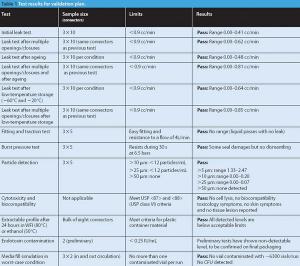
Sterile liquids are frequently transferred during the processing of sterile liquid drugs such as injectables or ophthalmic drops. Several types of transfer can be performed, each requiring a validated method to ensure the desired sterility-assurance levels are achieved.
Manufacturers use various techniques to transfer sterile liquid. Some methods, however, cannot accommodate disposable equipment, and others cannot transfer through barriers. This article describes a new approach for aseptic fluid transfer that was developed to provide a high-quality aseptic connection and simplify passage through a wall. The authors discuss the product-qualification results for the approach, which show that the technology and its various components meet pharmacopeial product-qualification requirements.

The closed vial has been developed to improve aseptic filling quality and to reduce process complexity. A ready-to-fill closed vial consists of a sterile vial provided with the stopper secured in place. The vial is filled by inserting a non-coring needle through the stopper, which is then resealed by laser.

Published: December 1st 2011 | Updated:

Published: June 2nd 2012 | Updated:

Published: December 1st 2009 | Updated:
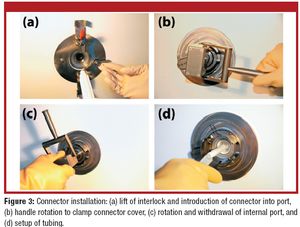
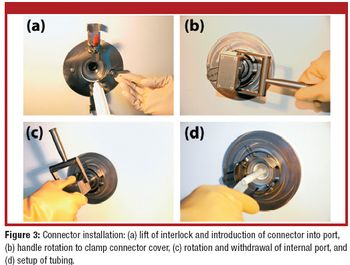
Published: April 2nd 2007 | Updated:
Published: June 1st 2007 | Updated:

Published: January 1st 2006 | Updated: