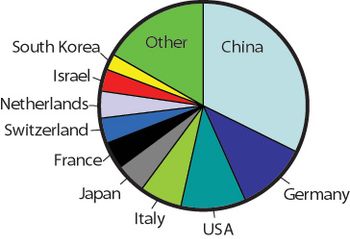
The author outlines the opportunities and challenges for manufacturers aiming to enter the BRIC-country markets.

The author outlines the opportunities and challenges for manufacturers aiming to enter the BRIC-country markets.

To meet the demands of early-stage development, contract research organizations can evaluate various dosage-form options. The author examines various methods of capsule filling, including binary blends.
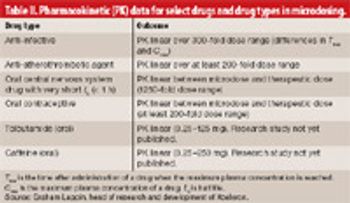
J. Scott Tarrant, executive vice-president of Xceleron, explains the role of microdosing in drug development. He describes how microdose data can be used to predict pharmacological dose absorption, distribution, metabolism, and excretion/pharmacokinetic outcomes using accelerator mass spectrometry.

Gonghua Pan, associate director and head of the parallel medicinal chemistry sourcing operations at Pfizer, explains the evolution of the company's approach to outsourcing research and development from a line-or function-centric approach to an integrated sourcing model. This analysis includes the role that contract research organizations in Asia play in the company's outsourcing actvities.

A sponsor company must investigate the manufacturing automation and systems, laboratory automation and systems, information-technology infrastructure, and business applications of each potential contract manufacturing organization.
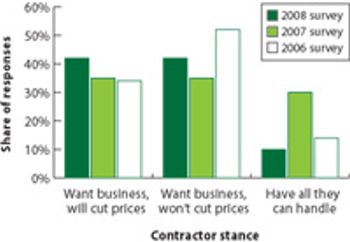
Survey results indicate a healthy market, but increasing competition.

Outsourcing strategies of large pharma companies are changing. The CEO of Patheon talks about his company's plan to meet customers' changing needs.

As pharma companies outsource new types of projects, contractors are trying to meet new needs.
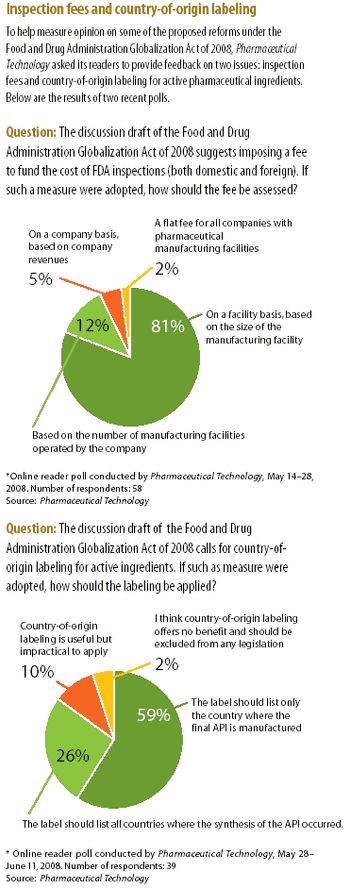
The high-profile case of contaminated heparin from a Chinese supplier has intensified the debate on the effectiveness of FDA's process for inspecting foreign drug-manufacturing facilities. The article examines proposed legislative and regulatory reforms and actions taken by the agency to improve drug-import safety.