
At a time when the industry is struggling with innovation, it might do well to learn a lesson from a few great innovators.
Michelle Hoffman, editorial director of Pharmaceutical Technology.

At a time when the industry is struggling with innovation, it might do well to learn a lesson from a few great innovators.

A report commissioned by FDA evaluates the QbD program.

Are biosimilars the next big thing or just the next big bubble?

Which route will we take to arrive at a national stem-cell policy?

Those who doubt there's faith in science, should check out our annual Bioprocessing Survey.
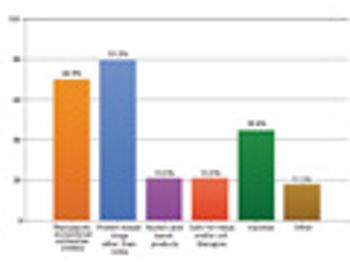
Results from our annual survey. This article contains online bonus material and is part of a special issue on Bioprocessing and Sterile Manufacturing.

Research and development may be headed for divorce.

Follow-ons were all the rage at this year's JP Morgan Healthcare Conference.

Capable of great works, pharma as a whole still yields to the lesser angels of its nature.

As we get ready to begin the New Year, let's hope some quality resolutions are made.
2010 came with pay raises for those still employed and anxiety for all. This article contains bonus online material.

A conversation with Mike de la Montaigne, president of Eisai Machinery, USA Inc., about the possibilities for conducting fully automated product inspections.

The reopened debate over embryonic-stem-cell research could stifle many other scientific pursuits.
Drugmakers hatch new manufacturing paradigms in the wake of the 2009 H1N1 influenza pandemic.

Novartis' Matthew Stober discusses vaccine manufacturing, including egg- and cell-based systems.

New data provide insight into pharma-industry professionals' daily lives.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

With more hands in the manufacturing pot, a good contingency plan is crucial to success.

After a spate of industrial disasters, the public seeks greater oversight of corporations-so does FDA.

The birth of "the pill" and harmonization created a new paradigm for global standards.

FDA wants industry to talk to them about the science underlying process innovations-really.

If both sides of the aisle don't agree on even mild healthcare reform soon, the bill could die out.
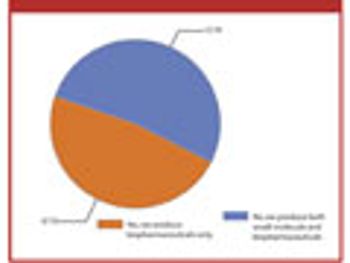
The second annual Pharmaceutical Technology Bioprocessing Survey offers a snapshot of the industry following 2009's megamergers.

A Q&A with GE Healthcare

The death of a pioneer in molecular genetics recalls age-old questions about social fitness to ensure the ethical uses of scientific advances.
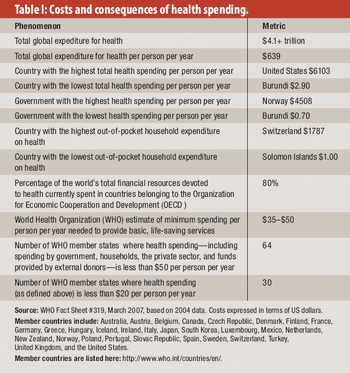
From healthcare to corruption to life expectancy, here's what we can learn from the past decade.

Results from our annual employment survey.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

A Conversation with Greystone Associates' George Perros. This article contains bonus online-exclusive material.

Published: November 2nd 2011 | Updated:

Published: August 2nd 2011 | Updated:

Published: July 2nd 2011 | Updated:

Published: September 2nd 2011 | Updated:

Published: April 2nd 2011 | Updated:
Published: May 1st 2011 | Updated: