
Editors' Picks of Pharmaceutical Science & Technology Innovations

Editors' Picks of Pharmaceutical Science & Technology Innovations
Information technology is the glue that should unify a company while ironically, it enables further fragmentation. Experts talk about the successes and challenges for IT in helping a company function efficiently.

Market demand for cytotoxic drugs is leading CMOs to expand their API manufacturing and formulation services.

The US Food and Drug Administration launched a new program on Oct. 4 to increase the number and variety of generic drugs available to the public, beginning in fiscal year 2008. The Generic Initiative for Value and Efficiency (GIVE) will use existing resources to help the agency "modernize and streamline the generic drug approval process," according to FDA.
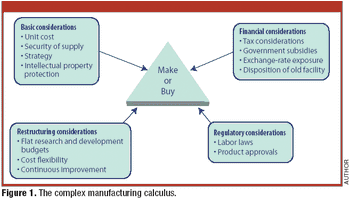
Candid comments from a Big Pharma executive highlight the complexity of contract manufacturing.

In this study, fault tree analysis applied to a water pretreatment and purification installation exposed cause-and-effect complex interrelations in possible fault events.
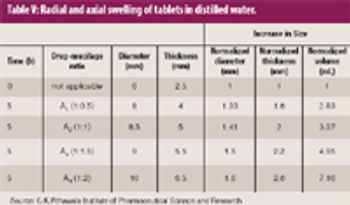
Natural gums and mucilage have been widely explored as pharmaceutical excipients. The goal of this study was to extract mucilage from the leaves of Aloe barbadensis Miller and to study its functionality as an excipient in pharmaceutical sustained-release tablet formulations.

Representatives from 17 nations sit down to decide upon standards-setting initiatives.

As the mother of a 15-month-old, I was quite shaken last month to hear about over-the-counter cold medicines for children under age 2 being pulled off the shelves. Glued to the news, I wondered if the decongestant I had given my daughter when she was sick would have any negative impact on her long-term health.

News and Views

Thin are the lines that separate stability, statistics, and chaos.

A book illustrates the potential for nanoparticulate drug delivery, and how much about them remains to be understood.

New FDA act reshapes drug development and marketing to restore public trust in pharmaceutical regulation.

Mixed-flow impeller systems exhaust laboratory workstation fume hoods, prevent reentrainment into the facility and adjacent facilities, and help companies comply with appropriate pollution-control standards.

Thanks to the media, Nobel Laureates are underrated, undervalued, and simply uncool.

The EIP process addresses the problems encountered with numerous questionnaires when qualifying excipient manufacturers.