
From pressurized metered dose inhalers to connected breath-actuated inhaler platforms and dry powder inhalers, Aptar Pharma’s c-Device solutions are developed to meet the unique data stream objectives of its customers

From pressurized metered dose inhalers to connected breath-actuated inhaler platforms and dry powder inhalers, Aptar Pharma’s c-Device solutions are developed to meet the unique data stream objectives of its customers

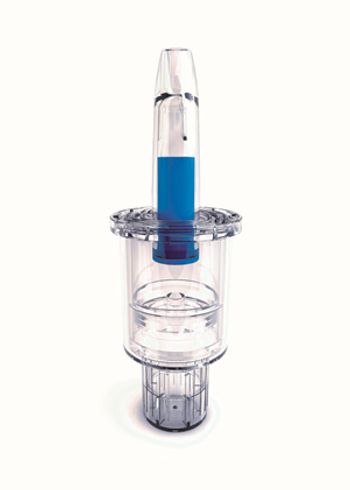
Aptar Pharma’s Unit-Dose Systems (UDS) are the preferred drug delivery technology spray platforms when dose accuracy and ease of administration are critical in a single nasal for liquid or powder drug delivery.
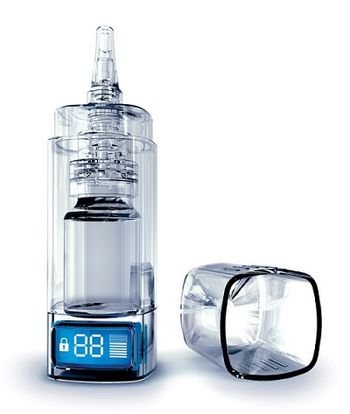
Aptar Pharma’s e-Lockout device is the first and only fully integrated electronic nasal drug delivery device to be approved by a US or European regulatory authority.

Aptar Pharma and Kali Care have announced that they entered into a partnership in order to address the challenge of monitoring adherence in ophthalmic clinical trials.


Published: March 14th 2017 | Updated:

Published: May 16th 2017 | Updated:

Published: June 13th 2017 | Updated:
Published: September 19th 2017 | Updated:

Published: October 24th 2017 | Updated:

Published: November 28th 2017 | Updated: