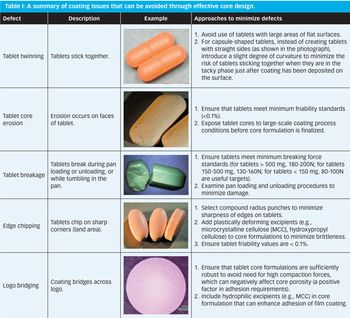
Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process.

Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process.

Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.

Suppliers must develop new technologies to drive the bio/pharma innovation engine.

Hazardous reagents can simplify processes and provide higher yields and purities.

Heightened global uncertainty could slow bio/pharma development activity.Bio/pharmaceutical companies, and the companies that serve them, tend to think they are immune from broader macroeconomic and political developments. As populations age, emerging middle classes expand, and scientific knowledge progresses, research on new drugs and demand for new therapies seem to follow an inexorably upward trend.

FDA and industry see progress and challenges in bringing cutting-edge medicines to patients.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss how to create a robust CAPA system and how to identify root cause.
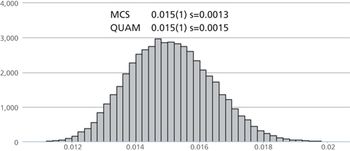
Performing a measurement uncertainty calculation is often seen as problematic.

Click the title above to open the Pharmaceutical Technology February 2016 issue in an interactive PDF format.

Sartorius Stedim Biotech’s (SSB) Sartoflow Smart is a benchtop crossflow system for optimized ultra- and diafiltration applications.

The Vacuum De-aeration Apparatus from Copley Scientific is used in semisolid pharmaceutical testing for de-gassing the medium used in vertical diffusion cells (VDCs) to measure the drug release profile of semisolid products.

Real-time GPS technology, better IT connections, and more conservative, controlled shipping temperatures are improving the shipment of sensitive pharmaceuticals.

The Ross X-Series is a clean-in-place (CIP) ready ultra-high shear mixer for inline emulsification, particle size reduction, and homogenization.

The Kimtech Pure A4 Sleeve Protector from Kimberly-Clark Professional features barrier protection that shields workers and the garment from hazardous chemicals and biologics.
The reporter bioassay, used to evaluate ADCC activity of therapeutic antibodies, complements the classical ADCC assay.

Today’s analytical laboratory equipment reflects the realities of downsizing, outsourcing, and the need for speed.

The EU’s key objectives include improving medicines access, tackling drug shortages, and increasing administrative efficiency in its regulatory framework.
A novel method, based on differential calculus, was used to calculate the maximum potential error associated with the drug concentration in pharmaceutical mixtures composed of an infinite number of ingredients measured on an infinite number of balances with different sensitivities. The method was further applied to calculate the ingredients’ least allowable quantities. This approach ensures that the pharmaceutical formulation is prepared within a given maximum permissible error in drug dose.