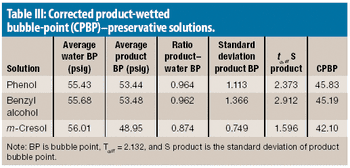
The authors explain the factors that can cause a failure in a bubble-point integrity test and what to consider when a product-specific bubble point must be defined.

The authors explain the factors that can cause a failure in a bubble-point integrity test and what to consider when a product-specific bubble point must be defined.
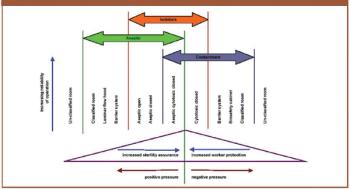
The author discusses the key issues to consider when using isolators such as containment, protection of personnel, the efficiency of biodecontamination cycles, sterility assurance levels, barriers and their integrity, and environmental impact.
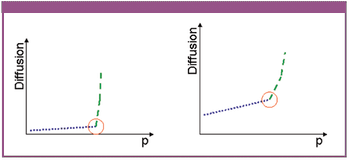
The authors describe a novel approach for the integrity testing of large sterile filter systems such as multiround housings and describe a multipoint diffusion test capable of detecting minor failures.
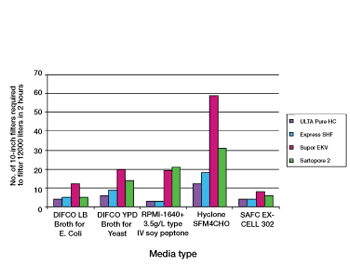
Proper selection of normal flow filters leads to increased process efficiency from early phase product development through to full-scale biopharmaceutical production.
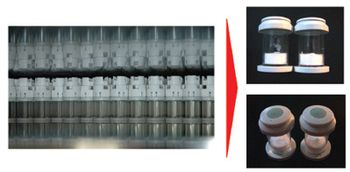
The authors present an aseptic-filling process for freeze-dried liquids using the closed-vial technology.

A comparison of conventional cleanrooms, restricted access barrier systems, and isolators, shows the benefits of using isolators in high-potency drug manufacturing.

Scott Sutton discusses the current state of USP ‹1117› and USP's plans for future revisions.
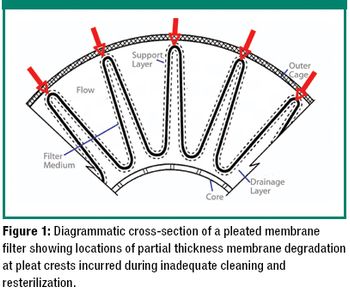
The author examines re-use of hydrophilic- or hydrophobic-membrane sterilizing-grade filters in liquid sterilizing applications.