There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.
Jerold Martin was the senior vice president of global scientific affairs at Pall Life Sciences and the chairman of the Board and Technology Committee at Bio-Process Systems Alliance.

There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

This column will address some of the questions on how single use systems are sterilized by gamma irradiation and what documentation may be requested by regulators.

Jerold Martin, senior vice president global technical affairs, at Pall Life Sciences, explains the importance and impact of single-use systems in sterile environments.

June and July saw three major US conferences on implementing single-use technologies: the IBC Single-use Applications meeting, the PDA Single-use Workshop and the Bio-Process Systems Alliance (BPSA) International Single-use Summit (ISUS). Jerold Martin highlights some of the key topics discussed at these meetings.

Strategies to minimise particle levels in the finished product when using single-use technologies downstream of final filters.

Regions around the world are starting to follow the US trend in disposables and are working to solve common challenges.

Over the past 10 years or so, we've seen single-use technologies explode from production-scale filter capsules, tubing and simple biocontainers to encompass sterile connectors, membrane chromatography adsorbers, bioreactors, mixers, and integrated platform systems with increasing levels of sensors and automated controls.

The development of quality agreements has long been recognised as a critical activity to ensure a product's quality meets regulatory requirements.

What have been the key innovations that have shaped current aseptic practices and techniques?
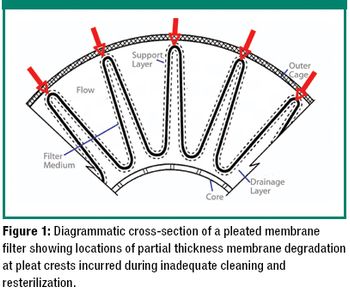
The author examines re-use of hydrophilic- or hydrophobic-membrane sterilizing-grade filters in liquid sterilizing applications.

Because disposable systems offer benefits that can impact the time for development as well as for the cost of production, the biotech sector is expected to continue to use these technologies ...

This paper discusses the most important factors that a filter user must take into account when making a filter selection.
Published: September 1st 2012 | Updated:

Published: November 1st 2011 | Updated:

Published: December 1st 2011 | Updated:

Published: February 1st 2012 | Updated:

Published: June 1st 2011 | Updated:

Published: September 1st 2011 | Updated: