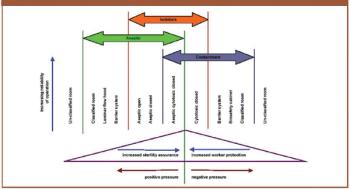
The author discusses the key issues to consider when using isolators such as containment, protection of personnel, the efficiency of biodecontamination cycles, sterility assurance levels, barriers and their integrity, and environmental impact.
Maurizio Battistini is the general manager of APP Pharmaceutical Partners in Switzerland.

The author discusses the key issues to consider when using isolators such as containment, protection of personnel, the efficiency of biodecontamination cycles, sterility assurance levels, barriers and their integrity, and environmental impact.

A comparison of conventional cleanrooms, restricted access barrier systems, and isolators, shows the benefits of using isolators in high-potency drug manufacturing.

Risk management is essential in any successful outsourcing partnership. The author outlines the steps toward identifying, understanding, and controlling risk in key manufacturing areas.
Published: November 1st 2008 | Updated:

Published: November 1st 2008 | Updated:

Published: August 1st 2007 | Updated: