
According to executive search firm Odgers Berndtson, its recent poll has highlighted that senior executives in life sciences believe there is a positive future for the United Kingdom’s sector outside the European Union.

According to executive search firm Odgers Berndtson, its recent poll has highlighted that senior executives in life sciences believe there is a positive future for the United Kingdom’s sector outside the European Union.

The National Institute for Health and Care Excellence (NICE) has issued guidance in Feb. 2020 stating that it does not recommend the combination therapy of pembrolizumab with axitinib for the treatment of advanced renal cell carcinoma in adults.

The Native Antigen Company has commercially introduced antigens that have been specifically derived from the Wuhan strain of novel coronavirus, now named Covid-19.

The report details OPQ’s accomplishments over the past five years.

Inhalation drug delivery company Vectura announces new organizational structure to drive innovation, customer focus, and growth.

Through the agreement, Catalent will offer process optimization and drug substance manufacturing services for the drug candidate at its Madison, WI site.

The acquisition will bring four new GMP-compliant facilities to the PCI network in the United States, Germany, and Canada.

Jim Walter will take on the role of vice-president of operations for Catalent’s Oral and Specialty Delivery business.

The Brand Security Platform from ACG Inspection uses blockchain, the Internet of Things, and artificial intelligence technologies to track packages from manufacturer to consumer.
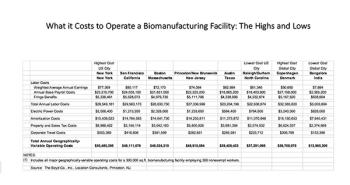
As politicians focus on drug cost reduction, biopharmaceutical companies in the US are moving to states with lower taxes, and relocating some facilities that had been offshore.

New funding brings competitors, and a leading healthcare products distributor, into the standardization effort.

The program supports Emerson’s digital transformation initiative which encourages employees to learn at a faster pace through immersive experiences that enhance safety and overall operational performance.
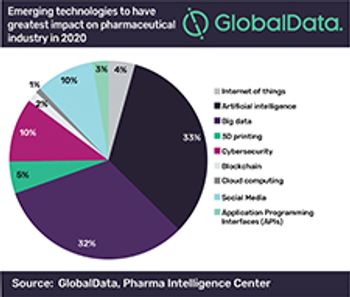
A growing number of pharma executives see investment in machine learning and big data as a top priority, according to a 2020 GlobalData survey.

The company will showcase the syriQ BioPure syringe in a 2.25-mL format for autoinjectors at Pharmapack in Paris, France from Feb. 5¬¬–6, 2020.

The research community is moving quickly to launch clinical trials of potential countermeasures, while regulatory authorities aim to support product development through regulatory flexibility.

The companies plan to provide characterization testing of container-closure systems for the biopharma industry.

Airnov announced on Feb. 5, 2020 at Pharmapack in Paris, France, that it will move forward as an independent company focused on desiccant and oxygen scavenging products for the pharmaceutical, diagnostic, and nutraceutical markets.

The two agencies are collaborating to support a robust biologics marketplace by taking steps to deter anti-competitive business practices.

FDA published draft guidance for applicants seeking licensure of a proposed biosimilar or proposed interchangeable biosimilar.

The new facility includes six classified environment rooms with space to expand.

Aimmune plans to introduce the antibody as an adjunctive treatment with its Characterized Oral Desensitized ImmunoTherapy programs to research treatment outcomes in patients with food allergies.

Catalent builds on its investment in cell and gene therapy development and manufacturing with the acquisition of MaSTherCell Global.

The knockout CHO K1 cell line will be used to support biotherapeutic R&D across a range of therapeutic indications.

West Pharmaceutical Services will be showcasing various new pharmaceutical containment and closure offerings at Pharmapack from Feb. 5–6 in Paris, France.

The FDA Commissioner plans to address drug prices, the drug approval process, and supply chain issues during his time as commissioner of FDA.

The vaccine is designed to provide active immunity against the influenza A (H5N1) strain and can be easily deployed in a pandemic event.

In replacing the retiring Paul Hegwood, Ricci Whitlow will oversee global clinical trial operations.

Bio/pharma researchers mobilize to diagnose and treat patients in pandemic threat.

The UK and Europe are entering a transitional period, which will involve negotiations across the board, including those on the pharma regulatory landscape.

Amid the rapidly rising cases of a novel coronavirus, concerns are being raised over preparedness and potential disruptions to the pharma supply chain.