
Lonza Plans Biologics Manufacturing Expansion
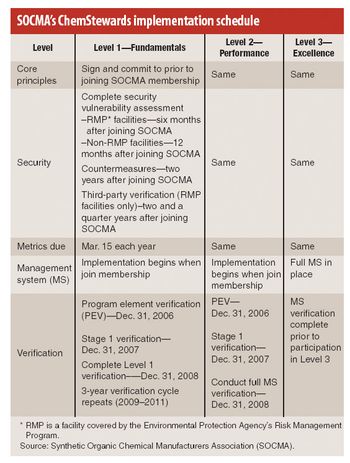
This is a year of change for the Synthetic Organic Chemical Manufacturers Association (SOCMA), the Washington, DC-based trade association representing chemical batch and custom manufacturers. Following the sale of Informex, its flagship trade show, last fall, the association is advancing key programs, most notably its new ChemStewards program, an environmental, health, safety, and security initiative (EHS&S) that its members began implementing last month.

Enterprise information integration delivers needed real-time capability to operational business intelligence.

SAFC Launches SAFC Supply Solutions

Eggs from Transgenic Hens Express Interferon Beta-1a Protein

Preliminary results from a clinical trial of Sanofi Pasteur's (Lyon, France, www.sanofi.com) H5N1 prepandemic influenza vaccine indicate the vaccine is safe and was well-tolerated in 300 healthy volunteers. This study is the first trial of an H5N1 prepandemic influenza vaccine candidate that compared vaccines with and without adjuvants.

Cell-Grown Vaccine Protects Against Avian Flu Virus

Non-Injectable Insulins Win Approval: Pfizer in the US, Generex in South America

If delaying the project has little consequence then you should probably not run the project in the first place.

It's all well and good providing drugs for people who need them, but it becomes a lot more difficult if they choose to ignore the warnings
The European Science Foundation (ESF) has published the conclusions of its 2-year Forward Look Study on Nanomedicine. Defined as a billionth of a metre, a nanometer is 1000 times smaller than the width of a human hair. Nanomedicine uses nanoscale technology to diagnose and treat disease. The scope of the study included defining the field of nanomedicine, reviewing what has been achieved so far, determining Europe's strengths and weaknesses, and drawing up plans to ensure continued growth.

Small- and medium-sized enterprises (SMEs) will gain administrative and procedural assistance from the EMEA. The agency's SME office was launched in December 2005 and follows the new Commission Regulation, which aims to promote the development of medicinal products in SMEs.

FDA Unveils New Prescription Drug Information Format

AAIPharma Moves Ahead with Reorganization Plan

FDA Eases Phase I Manufacturing Requirements

Warning: Brazilian Diet Pills Found to Contain Active Drug Ingredients

Pfizer Combats Counterfeiters with RFID

FDA Issues Dispute–Resolution Guidance

Novartis Considers Acquiring Berna Biotech

Schering-Plough Meets FDA Deadline for CGMP Improvements

Sanofi Pasteur and BD Team Up to Produce Microneedle Vaccines

Hubert J.P. Schoemaker Dead at 55
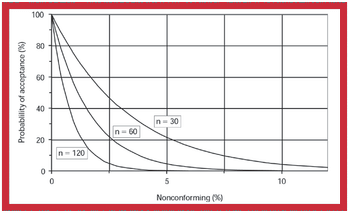
The benefits of zero tolerance as a test criterion have been oversold. A critical examination of zero tolerance reveals that many of the supposed benefits are not attainable. More important, inappropriate application of this criterion can have a deleterious effect on the assessment, control, and improvement of the quality of pharmaceutical products.

Is the lack of finance necessarily spelling doom for the European biotech industry?

Will we be innovative enough if we just become service providers?

Recent EU legislation change has opened the door for UK firm Accentus to use its novel predictive crystallization technology to discover and develop generic alternatives to patented drugs. Accentus will also licence its CrystalGEM predictive crystallization technology to generics manufacturers.

Pharmaceutical industry regulation is about to change with a new code of practice. This has been revealed by the Association of the British Pharmaceutical Industry (ABPI) following recent problems with patient safety and the dissemination of data and information of approved drugs, which exposed loopholes within the code.

Is the recent round of Big Pharma plant closings a sign of regrowth?

It's time for the pharmaceutical industry to consider electronic innovations as part of their life cycle management strategies.
Merck (Whitehouse Station, NJ, www.merck.com) has revealed the "first phase" of its global restructuring program set to eliminate 7000 jobs (11% of its global workforce) by the end of 2008, close or sell 5 of its 31 manufacturing facilities, and roll out a manufacturing strategy to "drive significant efficiencies, decrease headcount, and reduce or refocus operations throughout the plant network and the entire manufacturing division." The company also expects to close one basic research site and two preclinical development sites.