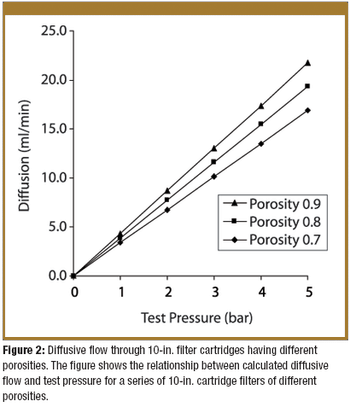
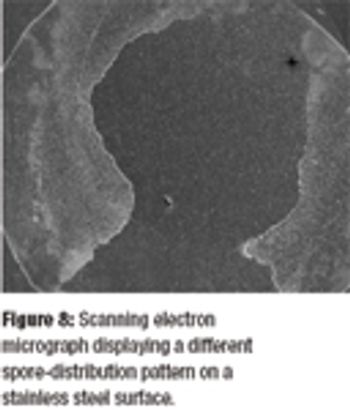
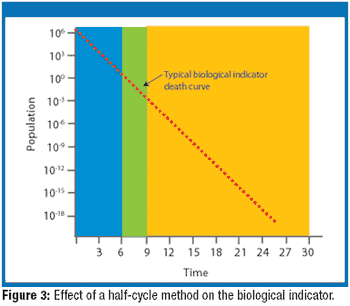
Irradiation is an established method of sterilization for pharmaceutical products. Radiation sterilization can be achieved with gamma rays, electron beams, and X-rays. Each of these techniques has its advantages and disadvantages. The author describes these methods, the ways to find the correct sterilization doses, and the regulatory and safety concerns about irradation sterilization.