
Microbial experts should employ proactive practices on the manufacturing floor, rather than relying on testing.
James P. Agalloco is the president of Agalloco & Associates, P.O. Box 899, Belle Mead, NJ 08502, tel. 908.874.7558, jagalloco@aol.com. He is also a member of Pharmaceutical Technology’s editorial advisory board.

Microbial experts should employ proactive practices on the manufacturing floor, rather than relying on testing.

Regulators have exaggerated expectations for simulated media fills.

Quality cannot be verified through testing, especially at the limit of detection, and no test method can confirm the absence of a microbe or particle.

A one-size-fits-all approach to monitoring practices and results is never appropriate, given the diversity of practice within the pharmaceutical industry.

Environmental monitoring, which operates below the limit of detection, is little more than a rote exercise that provides limited real value. So why does it figure so prominently in current regulatory requirements for aseptic manufacturing?

A previously published article presented difficulties with the revised European guidelines on sterile manufacturing. The authors included a brief summary of the comments developed on the draft document. This article expands upon that summary, outlines the authors' rationale, and highlights the most difficult aspects of the revision draft.

The following study describes a methodology to establish, control, and characterize an aerosolized bioburden within a test chamber and determine the settling rate of the bioburden.

The revised Annex 1 on sterile manufacturing includes incorrect and ambiguous statements that must be fixed before implementation.

Alternatives to time-consuming, error-prone operations promise to reduce vaccine manufacturing costs and improve facility flexibility.

The revised Akers-Agalloco aseptic risk assessment and mitigation method includes two sub-methods that can be used independently for risk assessment.

Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

The half-cycle method for validating sterilization can have adverse effects on materials if used for steam sterilization.

This article discusses the issue and offers food for thought and suggestions for more science-driven approaches.

Microorganism lethality requirements for process validation must always be balanced with the need to protect product integrity and patient safety.
The Human Microbiome Project has increased our understanding of the relationship between humans and microorganisms. The authors offer a new perspective on how this knowledge should be considered in setting standards for pharmaceutical quality control in microbiology.
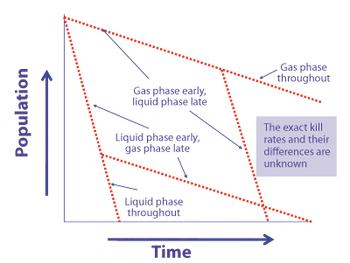
Vaporous hydrogen peroxide, used for sterilization and decontamination, is highly potent but presents implementation challenges.
The authors assert that the current gulf between aseptic processing and terminal sterilization can be bridged by re-examining fundamental regulatory philosophies for sterile-product manufacturing.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

The authors revisit their previous effort to refine the terms that describe interventions and to dispel confusion that arose after the original article was published.

The author describes why statistical significance would impose an unreasonable burden on manufacturers.

The author explains the idea of equivalence and describes how it can facilitate equipment validation.

What are the current expectations for cleanroom operations? And are these realistic?

The authors propose a process-centric approach for carrying out aseptic-processing and suggest further dialogue. This articles contains bonus online-exclusive material.

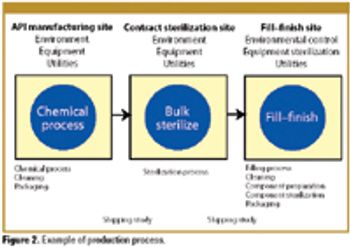
The author describes various manufacturing processes and evaluates whether the guidance can be applied to each of them.
Patient safety must be the primary concern of any validation effort. The author explains how a risk-based approach to validation and compliance follows naturally from this premise.

Preparing sterile products requires manufacturers to control microbial quality. Sterility and endotoxin content are critical because failure to properly manage them can seriously harm, or even kill, patients.
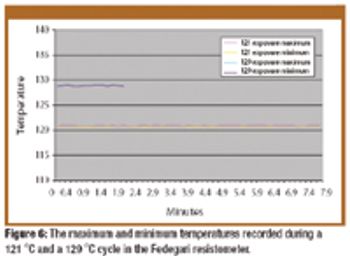
The authors examine advances in the design and the application of biological indicator evaluator resistometer vessels used to measure the resistance of bacterial spores in monitoring sterilization processes.
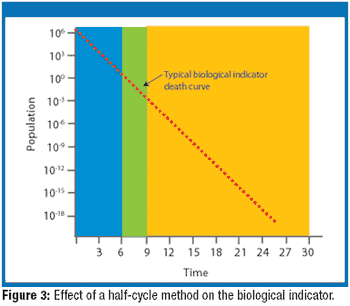
The author clarifies the definition and objectives of overkill sterilization for steam sterilization cycles. Current sterilization practices are reviewed and the validation difficulties associated with the various definitions of overkill sterilization are explored.

Aseptic processing has advanced over the past several decades, yet the pharmaceutical industry is still accepting of its limitations, particularly as it relates to human intervention as a source of contamination. The authors explain the importance of further diminishing the role of operators in aseptic processing and the approaches and technologies needed to achieve that goal.

This article summarizes changes to the Akers–Agalloco aseptic processing risk analysis model (first presented in Pharmaceutical Technology's November 2005 issue) as well as some of the underlying thinking behind the revision. The simplified model makes the method easier to use because of its greater flexibility of environmental control practice. It maintains the emphasis on human activity as the primary consideration in risk management for aseptic processing.

Published: September 2nd 2021 | Updated:

Published: November 2nd 2001 | Updated:

Published: October 2nd 2001 | Updated:
Published: September 2nd 2013 | Updated:
Published: May 1st 2012 | Updated:

Published: May 1st 2011 | Updated: