
A one-size-fits-all approach to monitoring practices and results is never appropriate, given the diversity of practice within the pharmaceutical industry.
James E. Akers is the president of Akers Kennedy & Associates, PO Box 22562, Kansas City, MO 64113, akainckc@aol.com.

A one-size-fits-all approach to monitoring practices and results is never appropriate, given the diversity of practice within the pharmaceutical industry.

Environmental monitoring, which operates below the limit of detection, is little more than a rote exercise that provides limited real value. So why does it figure so prominently in current regulatory requirements for aseptic manufacturing?

A previously published article presented difficulties with the revised European guidelines on sterile manufacturing. The authors included a brief summary of the comments developed on the draft document. This article expands upon that summary, outlines the authors' rationale, and highlights the most difficult aspects of the revision draft.

The revised Annex 1 on sterile manufacturing includes incorrect and ambiguous statements that must be fixed before implementation.

The revised Akers-Agalloco aseptic risk assessment and mitigation method includes two sub-methods that can be used independently for risk assessment.

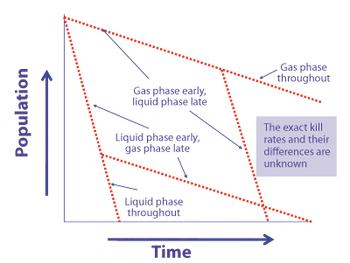
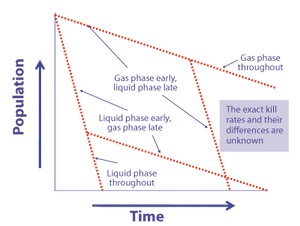
Microorganism lethality requirements for process validation must always be balanced with the need to protect product integrity and patient safety.

The recovery of an occasional mold does not merit any particular concern. On the other hand, evidence of mold proliferation indicative of infection of facilities or equipment must be taken seriously and requires the prompt implementation of corrective and preventive actions.

Understanding how to identify, remediate, and prevent facility infection is crucial for product quality.
Vaporous hydrogen peroxide, used for sterilization and decontamination, is highly potent but presents implementation challenges.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

The authors revisit their previous effort to refine the terms that describe interventions and to dispel confusion that arose after the original article was published.

The updated edition of William Whyte's book provides information for novices and seasoned professionals alike.

The authors propose a process-centric approach for carrying out aseptic-processing and suggest further dialogue. This articles contains bonus online-exclusive material.

The authors review the role of automation in aseptic processing and describe their experience in implementing advanced technologies, including the use of isolators and robotics.
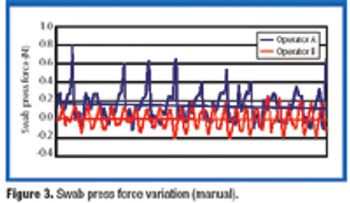
This article presents a study of an aseptic environmental monitoring system for surface contamination at critical areas using a robot.

Sterile product manufacturing and related testing have evolved significantly during the last 30 years. From requirements for acceptance criteria for media-fill tests, to developing validated approaches for moist-heat sterilization, to the introduction of formalized sterility-testing practices, the pharmaceutical industry has made significant advances in testing and in key technology such as isolators, prefilled syringes, automation, and robotics. The author outlines the key regulatory and technical changes to sterile product manufacturing and takes a visionary look for the next era of sterile manufacturing marked by a greater emphasis on risk analysis.

Aseptic processing has advanced over the past several decades, yet the pharmaceutical industry is still accepting of its limitations, particularly as it relates to human intervention as a source of contamination. The authors explain the importance of further diminishing the role of operators in aseptic processing and the approaches and technologies needed to achieve that goal.

This article summarizes changes to the Akers–Agalloco aseptic processing risk analysis model (first presented in Pharmaceutical Technology's November 2005 issue) as well as some of the underlying thinking behind the revision. The simplified model makes the method easier to use because of its greater flexibility of environmental control practice. It maintains the emphasis on human activity as the primary consideration in risk management for aseptic processing.
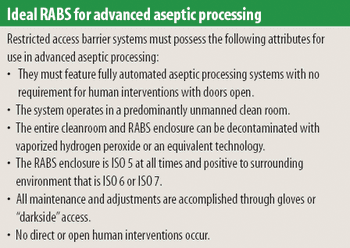
Any aseptic processing technology that allows intervention by gowned personnel during operation cannot be considered an advanced technology. Although a standardized definition of restricted access barrier systems has been developed, these systems fall well short of being classfied as advanced technologies.
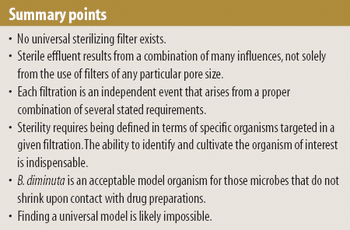
Model organisms are useful when validating sterile filtration, but successful retention of the model organism does not always guarantee that effluent is sterile. The authors explore the various factors that influence sterile filtration.

The industry has acknowledged only recently the significance of the contamination risk posed by humans. The authors assert that this realization, together with technological advances, will lead to the elimination of human intervention and, hence, improved sterility.

The authors present a new approach to risk assessment for aseptic processing that emphasizes the contributions of personnel.

The complete elimination of human-derived contamination is possible only with the elimination of human intervention.
Published: September 2nd 2013 | Updated:

Published: May 1st 2011 | Updated:

Published: April 2nd 2011 | Updated:

Published: August 2nd 2010 | Updated:

Published: March 1st 2010 | Updated:

Published: May 1st 2009 | Updated: