
Q&A with David Elder of Strategic Compliance Consulting, PAREXEL International on preparing for the rise in inspections. Elder is a former senior official with FDA.

Q&A with David Elder of Strategic Compliance Consulting, PAREXEL International on preparing for the rise in inspections. Elder is a former senior official with FDA.

Careful attention to detail will help to prevent valuable assets from "melting" away.

Taking time to appreciate the industry's greatest achievements will inspire growth ahead.
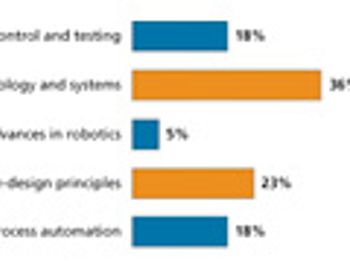
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

A Q&A with FDA Deputy Commissioner Deborah Autor.

An industry roundtable on how users and makers can best assess and manage black specks.

Applying current principals to traditional factorial designs.

Uniform dose formulation is key to meeting safety study requirements.

Presidents of leading associations offer views on the industry's future.

FDA has updated its website to include the latest Warning Letters issued to pharmaceutical companies by the Office of Prescription Drug Promotion and the Center for Drug Evaluation and Research.

The European Medicines Agency's Committee for Orphan Medicinal Products made recommendations for nine orphan drug designations during its June 2012 meeting. Included in COMP's recommendations were four designation applications for rare forms of lipodystrophy.

Basilea, Stiefel Enter into Hand-Eczema Pact; Sandoz Recalls Generic Oral Contraceptive Drug; and More.

Are pharmaceutical manufacturers really serious about ensuring the quality of their medicines? And do they recognize that cutting corners on controls and quality management can be tremendously costly in the long run?

European and US associations call for continued vigilance against the threat of counterfeit medicines.

In July 2012, new pharmacovigilance legislation will come into effect across the EU as a result of changes adopted in 2010, specifically EU Regulation No. 1235 and Directive 84.

The United States Pharmacopeia has stated that references to General Chapter <911> "Viscosity" will be changed to General Chapter <911> "Viscosity-Capillary Viscometer Methods," <912> "Rotational Rheometer Methods," or <913> "Rolling Ball Viscometer Method."

Q&A with Peter Smith, Strategic Compliance Consulting, PAREXEL International, and a former senior official with FDA, on change management best practices.

IQ Consortium representatives explore and define common industry approaches and practices for applying GMPs in early development.

BIO is calling for a more patient-centric approach to user-fee reauthorization.

US Pharmacopeia documents best supply-chain practices and seeks broad input on proposal.

New price-control policy has domestic and global firms waiting on the sidelines to launch products.

New legislation, government programs aim to bolster drug discovery and reduce regulatory hurdles.

Does global development have to entail multiple comparability studies?

Even the slightest of errors in exponential calculations can cause the biggest of headaches.

The EDQM (European Directorate for the Quality of Medicines and HealthCare) are looking to recruit a Scientific Programme Officer to join the team for a five year period to organise, manage and monitor a number of projects related to Pharmaceutical Care and Anti-Counterfeiting activities with a particular focus on the implementation of the MEDICRIME Convention