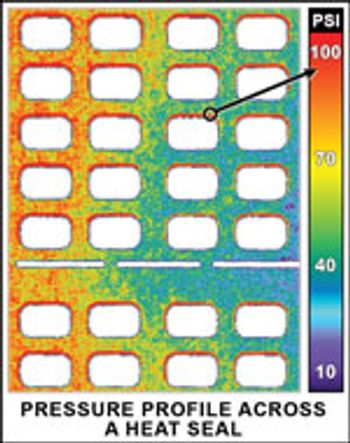
Equipment and Processing Report
The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.