Equipment
Latest News

Latest Videos

More News

With advanced manufacturing, BioPure’s BioClamp connector is manufactured to be 13% lighter than the previous model, resulting in a 26% reduction in carbon dioxide emissions across the full lifecycle of the product.

Industry 4.0 is driving adoption in the pharma industry for smart equipment and tools that will advance manufacturing.

The company says it is the pharmaceutical market’s first wet granulator with an integrated, truly continuous dryer.

The European Commission has approved Novo Holdings' acquisition of Catalent, which includes the related sale of three manufacturing sites to Novo Nordisk, which is also acquiring the Czech Republic manufacturing site of Novavax for $200 million.

The ROSS Model 42N-10 has a working capacity of 10 cubic feet and is designed for free-flowing materials with bulk densities up to 100 lbs per cubic foot.

SEKISUI has used a £15.7 million (US$20.7 million) investment to expand its clinical-grade drug substance manufacturing at its UK site.

Financing from OrbiMed, Novo Holdings, Jeito Capital, and others will enable Alentis Therapeutics to advance its pipeline of Claudin-1-targeted ADCs for treating solid tumors.

Evolving industry demands are driving the need for greater flexibility and innovation in processing equipment for pharmaceutical manufacturing.

Shifting to automated, closed modular manufacturing systems is growing increasingly crucial for biopharmaceutical production.

The ROSS FDA-50 Fixed Tank Dual Shaft Mixer is built to handle a wide range of formulations and viscosities and is ideal for processes that require meticulous control over mixing, temperature, and pressure.

The Mobius ADC Reactor is the first single-use reactor designed specifically for the manufacture of antibody drug conjugates.
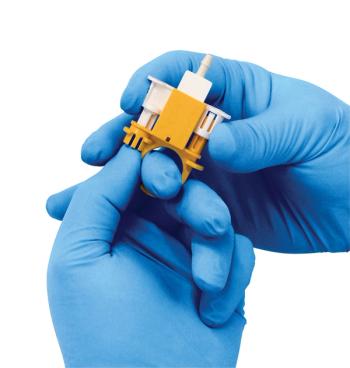
CPC’s MicroCNX ULT Series represents the first aseptic microconnector that can be fitted directly into freeze cassettes for GCT production.

According to the annual survey, four of the five biggest biologics capacity holders in 2028 will comprise CMOs, which are expected to have 45% of all CMO capacity in Asia.

As part of a range of sustainable products and services that SGD Pharma plans to showcase at CPHI Milan, the company will introduce its siliconized molded glass vials.
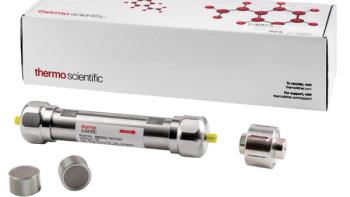
Thermo Scientific’s columns offer fast run times, optimal separation efficiency, and reliable results.
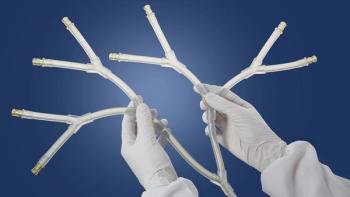
Freudenberg Medical’s new offerings include single-use Y-connector manifolds, tubing assemblies, and bottlecap assemblies, among others.

Charles Ross & Son Company has introduced the ROSS CDA-300 Dual Shaft Mixer, consisting of two agitators and a disperse with an open disc blade.

Asahi Kasei Medical now offers its Planova virus removal filters for use in biopharma manufacturing and plasma derivatives manufacturing.

The new additions to the company’s equipment at its early phase clinical manufacturing facility in Oregon will allow for cleaning, filling, capping, and sealing, and also detecting defects in drug products.

Freudenberg Medical has introduced custom single-use assemblies for silicone extrusion and molding for biopharma applications.

The ROSS FDA-3500 dual shaft mixer is equipped with independently controlled drives and is designed to efficiently produce good turnover and impart shear to a viscous batch.

The NexGen Press 45 is the latest member of GEA Pharma & Healthcare’s NexGen Press line of tablet presses.

Syngene’s protein production platform uses a cell line and transposon-based technology licensed from Switzerland-based ExcellGene.

Univercells and the University of Pennsylvania are expanding their collaboration to evaluate certain bioreactors with the aim to scale up gene therapy manufacturing.

Ecolab Life Sciences and Repligen have launched a new affinity resin, DurA Cycle, for large-scale biologics manufacturing.