Regulatory Consulting
Latest News
Latest Videos

More News

Pharmalex has launched the Biopharma Excellence brand, a new service line that combines the expertise of PharmaLex, ERA Consulting, and Biopharma Excellence.

ProductLife Group (PLG) and Juuka Advisory have teamed up to form a new consulting offering, ProductLife Consulting.

The company now offers its CONFIDENCE virus clearance services to support validation of viral clearance processes.

Data governance is necessary for compliance with current regulatory expectations for data integrity in pharmaceutical R&D and manufacturing organizations.

Regulatory outsourcing can result in improved compliance, greater transparency, higher productivity, increased cost-effectiveness, and desired strategic outcomes.

A robust quality agreement and good communication scheme can help avoid and alleviate regulatory concerns.

Pharmaceutical Technology asked Siegfried Schmitt, principal consultant at PAREXEL, about the importance of quality agreements in the sponsor/contractor relationship.

More life-sciences companies are starting to manage global suppliers holistically.

Although both sponsor and contract partner must comply with quality regulations, regulators say the final responsibility for quality lies with the sponsor

Quality, innovation, and new approval pathways open drug development options for the Chinese market, including injectable contract manufacturing.

Training and mock audits are the key to preventing data integrity issues with partners offshore, but the process must start at home. Compliance consultant John Avellanet shares best practices and ways to minimize costs.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to report quality metrics to FDA.

Transparent communications, both qualitative and quantitative data, and a clear understanding of each other’s needs are keys to collaborating on better product quality.

*This article is an opinion piece and does not necessarily represent the views of BioPharm International.

Contract service providers describe how quality by design has influenced a drug sponsor's expectations of suppliers.
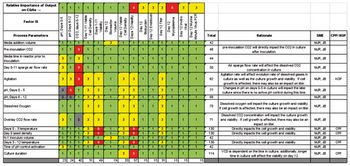
Traditional project decision-making is compared with a QbD approach.

Recent draft guidance from FDA on contract manufacturing and quality agreements highlights the importance of such agreements and define the roles and responsibilities of each party to be in keeping with quality risk-management principles.

The author discusses how a CDMO helps in gaining process understanding and in developing robust, high-quality products and processes.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.