Manufacturing, Gene Therapies
Latest News

Latest Videos
More News

Through this global alliance, PackGene, Weill Cornell Medicine, and GC4K, an Australian non-profit, intend to deliver a custom-tailored gene therapy solution to treat hereditary spastic paraplegia type 56, a particularly rare neurological disease.

EXO Biologics and ExoXpert, an EXO Biologics subsidiary, have received GMP certification of a European manufacturing facility for exosomes and have successfully loaded mRNA and DNA payloads into GMP-grade exosomes for drug delivery.

Manufacturers of five autologous or matched allogenic cell therapy products have selected TrakCel's IT platform, OCELLOS, to orchestrate the administration of these therapies, which are approved or expected to be approved in 2024.
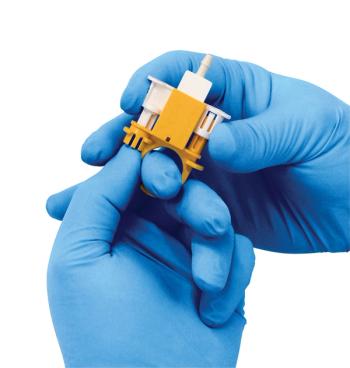
CPC’s MicroCNX ULT Series represents the first aseptic microconnector that can be fitted directly into freeze cassettes for GCT production.

SK pharmteco will be the preferred manufacturing partner for AVG-101, AaviGen’s lead gene therapy product.

The nanofiber microcarriers are the first of their kind for manufacturing viral vectors used in gene therapy production, according to Cellevate.

The two companies are partnering to provide logistics and manufacturing services to biotechnology and pharma companies.

Lonza signed a long-term supply agreement to manufacture CASGEVY (exagamglogene autotemcel) for Vertex at its facility in Geleen, the Netherlands, and plans to expand manufacturing to Portsmouth, NH, in the United States.

The next-generation IRO platform was found to significantly outperform the legacy Prodigy system, showing improvements in cell growth, CAR+ cell yield, and overall efficiency.

The collaboration is designed to combine NanoVation Therapeutics’ lipid nanoparticle technology for RNA delivery to cells outside the liver with Novo Nordisk’s knowledge of cardiometabolic and rare disease R&D.

Viral vectors and other complex biologic modalities require more specificity and higher sensitivity to detect and distinguish contaminants.

Rentschler Biopharma has expanded its service offerings at its Stevenage, UK, site with a new lentiviral vector manufacturing toolbox.

With the agreement, Andelyin Biosciences will add MyoAAV plasmids developed by Broad Institute of MIT and Harvard to its AAV Curator Platform.

BioIVT opened new cleanroom manufacturing space, using Germfree’s technology to boost its capabilities for cell and gene therapy development.

Innovative solutions are making personalized cell and gene therapies accessible to all.

Univercells and the University of Pennsylvania are expanding their collaboration to evaluate certain bioreactors with the aim to scale up gene therapy manufacturing.

The acquisition of uniQure’s Lexington, Mass., manufacturing operations will boost Genezen’s late-phase and commercial gene therapy development and manufacturing services.

Sarepta Therapeutics received expanded approval from FDA for Elevidys in the treatment of DMD in non-ambulatory patients as ell as ambulatory patients.

Lonza’s Joe Garrity and Jerry Jiang discuss the importance of not only automating CGT manufacturing, but also standardizing across processes.

Lonza’s Joe Garrity and Jerry Jiang discuss the latest trends and challenges in commercializing new CGTs.

CGT Catapult and CATTI aim to standardize advanced therapy manufacturing with new aligned training standards.

Pharm Tech Group chats with Dr. Monika Paulė, CEO and co-founder of Caszyme about the evolution of CRISPR.

CGT Catapult will implement Cellular Origins' robotic platform in its Stevenage, UK, site to establish automated CGT manufacturing.

With this acquisition, Merck KGaA will have the capability to offer integrated services for viral vector manufacturing.

The separation of deamidated capsids from non-deamidated capsids can be achieved using HIC, according to studies done by a team at Oxford Biomedica.