
The collaboration between Samsung Biologics and Pfizer will focus on long-term biosimilars manufacturing.

The collaboration between Samsung Biologics and Pfizer will focus on long-term biosimilars manufacturing.

The new partnership between Sandoz, a Novartis division, and Just – Evotec Biologics will aim to develop and manufacture multiple biosimilars.

Sandoz, a Novartis division, is expected to invest at least $400 million to build a new biologics manufacturing facility in Lendava, Slovenia, to support growing demand for biosimilars.

The commercialization and licensing deal will see Biogen commercialize Xbrane’s Xcimzane, a proposed biosimilar for CIMZIA (certolizumab pegol).

Samsung Biologics has signed an agreement with Biogen to acquire Biogen’s 50% stake in Samsung Bioepis, a joint venture formed by the two companies, for up to $2.3 billion.

Sandoz will soon begin enrolling patients with neovascular age-related macular degeneration in a Phase III study with its proposed biosimilar to aflibercept.

The companies received approval from the EC for Zercepac (HLX02), a biosimilar referencing Roche’s originator biologic, Herceptin (trastuzumab).

AzarGen’s biosimilar, made in iBio’s plant-based system, will be compared to the original molecule in pre-clinical studies.

The partnership will provide cost effective biologics for the world market.

A global agreement with Polpharma Biologics gives Sandoz commercialization rights to a proposed biosimilar natalizumab for relapsing-remitting multiple sclerosis.

The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.

Experts believe that the contract development and manufacturing organization market will reach $17.38 billion by 2022, with disruptive business models using Industrial Internet of Things (IIot) and single-use technologies proving more profitable and efficient in the long term.

Specialist biopharmaceutical company, Alvotech, has announced receipt of a manufacturing license from the Icelandic Medicines Agency, applying to its biopharmaceutical facility based in Reykjavik, Iceland.

While food, shelter, and clothing are the primal essentials for life, hope-as embodied by modern medicine-has now become part of that human expectation.

The collaboration will focus on developing manufacturing solutions for biosimilars.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.
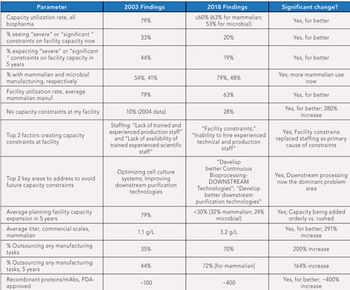
This article highlights 15 years of changes in biopharmaceutical manufacturing.

Macromolecular drugs are typically injected, but oral dosage forms are being developed to improve the treatment of gastrointestinal conditions such as inflammatory bowel disease.

This marks the third FDA approval for the company’s second biomanufacturing plant in Incheon, Korea.

The European Commission has approved Zessly (infliximab), a biosimilar to Johnson & Johnson’s blockbuster Remicade (infliximab).

Valerius Biopharma will use Catalent’s GPEx technology to produce cell lines for biosimilar drugs.

Celltrion received complete response letters from FDA for its rituximab and trastuzumab biosimilars.

The companies have created Syna Therapeutics, a joint venture that will develop biosimilars and new molecules.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.