Pharmaceutical Technology Europe
Monoclonal antibodies and recombinant proteins have increased their importance and gained success as therapeutic agents in the treatment of various diseases.

Pharmaceutical Technology Europe
Monoclonal antibodies and recombinant proteins have increased their importance and gained success as therapeutic agents in the treatment of various diseases.

Pharmaceutical Technology Europe
Most CROs are entering China with expectations that a significant local market opportunity will develop as major pharmaceutical companies establish research development operations there...

Pharmaceutical Technology Europe
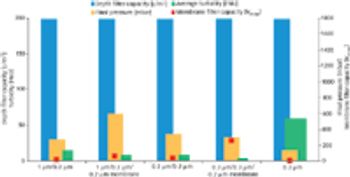
As the US biopharmaceutical industry and regulators debate new requirements for biosimilars, industry leaders are turning to analytical science to define intellectual property and business development strategies. Emerging techniques are providing previously unseen protein characterization details, giving both innovator and generics companies new weapons in the battle for future market share.

Pharmaceutical Technology Europe
It is the end of an era. Building on more than 6 years of dedication and hard work, Gurminder Marwaha has left Pharmaceutical Technology Europe (PTE) in search of new challenges. As his successor, I would like to join all the members of the editorial team in wishing him every success.
Pharmaceutical Technology Europe
Biopharmaceuticals are the most rapidly growing segment of the pharmaceuticals market. Developing and marketing biopharmaceuticals are huge roles in almost every major pharmaceutical company's strategy. However, they are extremely complex molecules and are highly sensitive to the manufacturing processes used to produce them. These processes require exquisite control of living production systems, making, without a doubt, biopharmaceuticals one of the most challenging products of any type to manufacture.
Pharmaceutical Technology Europe
The blood–brain barrier (BBB) forms an interface between the circulating blood and the brain, and functions as a tremendously effective barrier for the delivery of potential neurotherapeutics into the brain parenchyma. Conversely, the BBB possesses various carrier-mediated transport systems for the uptake of small molecules, such as essential nutrients and vitamins. These transporters have become an attractive target for drug/prodrug design in an attempt to ferry drug molecules across the BBB. Central nervous system (CNS) drug delivery is often limited by poor brain penetration of the potential drug candidate. As a result of its unique barrier properties, the BBB poses a huge challenge for the delivery of potential neurotherapeutics into the brain parenchyma.1 It is estimated that only 2% of small-molecule drugs and ,0.1% of novel protein and peptide pharmaceuticals developed for CNS diseases reach therapeutic concentrations in the brain.2,3 Many of the pharmacologically active drugs tend to fail..

Pharmaceutical Technology Europe

Pharmaceutical Technology Europe
Manufacturing facilities must be inspected by members of regulatory bodies. However… these bodies are woefully inadequate at performing the task.

Pharmaceutical Technology Europe
The amount of water used by industry, including pharma and biotech manufacturing, amounts to 23% of the world's supplies
Pharmaceutical Technology Europe
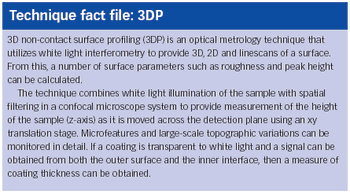
Our company is involved in developing and manufacturing APIs that can be utilized with drug-eluting stents (DES). Despite ensuring constancy in pharmaceutical composition, we are experiencing issues with variations in drug release during in vitro studies. We are working closely with a stent manufacturer to develop the system, but could surface analysis techniques investigate the problem further?
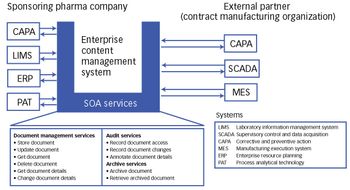
Pharmaceutical Technology Europe
As a skipping stone creates ripples in a lake, SOA can help create benefits that quickly ripple through many other areas of the organization and partners.
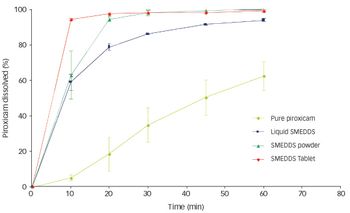
Pharmaceutical Technology Europe
Spraying techniques can be used to produce powder form formulations. The concept works by the adsorption/absorption of a liquid SELF onto a neutral carrier…