
Pharmaceutical Technology Europe
The different pathways to regulatory approval of a biosimilar vary worldwide, ranging from no pathways at all in some developing countries, to the complex and precise mechanism that exists in Europe.

Pharmaceutical Technology Europe
The different pathways to regulatory approval of a biosimilar vary worldwide, ranging from no pathways at all in some developing countries, to the complex and precise mechanism that exists in Europe.

Pharmaceutical Technology Europe
Many pharmaceutical companies in the UK have adopted a direct-to-pharmacy distribution model, which enables companies to more tightly control their supply chains.

Pharmaceutical Technology Europe
Overall Equipment Effectiveness has been shown to identify the root cause of inefficiencies.

Pharmaceutical Technology Europe
A new guideline for conducting bioequivalence studies was adopted by the CPMP in January and it becomes fully effective as of August 2010.

Pharmaceutical Technology Europe
We speak to The European generic Medicines Association about the environment for biosimilars.


Pharmaceutical Technology Europe
The interest in developing biosimilar medicines has grown dramatically in recent years with biotech drugs gradually increasing their share of the overall therapeutics market worldwide to account for an attractive portion of the sales pie.

Pharmaceutical Technology Europe
The recent amendment to Annex 1 has seen controversial changes relating to the capping of vials.

Pharmaceutical Technology Europe
New isolator and disposable technologies are set to assume a greater role in pharma manufacturing, according to a recent conference in Germany.

Pharmaceutical Technology Europe
The EMA's Guideline on Similar Biological Medical Products provides overarching guidance, but since its introduction a range of more specific guidelines have also been developed.

Pharmaceutical Technology Europe
As biopharmaceuticals will soon make up 50% of new drug approvals there has been a significant rise in interest in the field of biosimilars.

Pharmaceutical Technology Europe
The biggest differences between the development of a biosimilar and a generic medicine lie primarily in the preclinical and clinical stages.

Pharmaceutical Technology Europe
Why the primary defence against biosimilars is to develop better molecules that can be launched quickly.
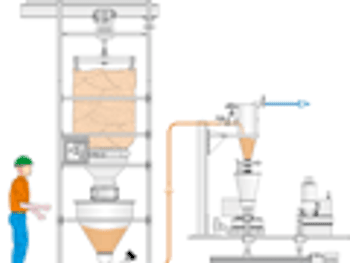
Pharmaceutical Technology Europe
What are the newest trends in pneumatic conveying of pharmaceutical powders and blends?