Pharmaceutical Technology Europe
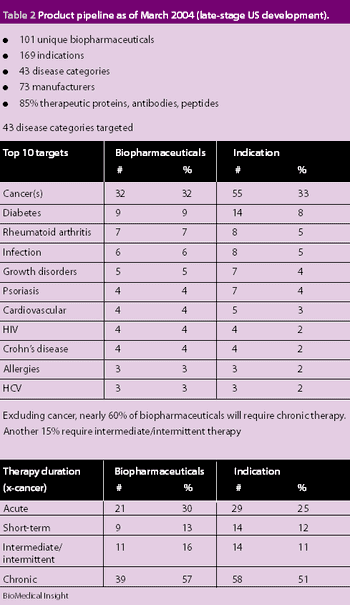
The worldwide market for biopharmaceuticals was estimated to be $50 billion in 2005. North America accounts for 60% in terms of revenue and R&D. Europe accounts for 20% and Japan 10%. It is also estimated that 400–500 biotech drugs are under clinical development for various disease conditions. Biopharmaceuticals are being developed to fight cancer, viral infections, diabetes, hepatitis and multiple sclerosis. The distinct families of biopharmaceuticals include



