
FDA’s focus on the quality culture and its request for quality metrics may ensure a successful company-CMO relationship.

FDA’s focus on the quality culture and its request for quality metrics may ensure a successful company-CMO relationship.

Cold-chain requirements and the tight logistical windows needed for cell and gene therapies demand a focus on early communication and risk mitigation.
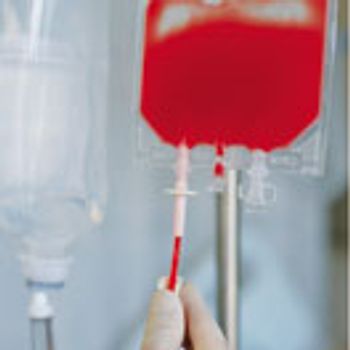
Andrea Zobel, senior director of product management, clinical trial supply, and logistics at PAREXEL International, examines key issues and questions that sponsors and contract partners must address.

Many pharmaceutical manufacturers were late in involving contract partners in serialization efforts. Are you ready, and are you working with the right partner?
As the date for implementation of the Drug Supply Chain Security Act approaches, bio/pharma companies and contractors should focus on key areas.

Selecting the best partner contract service and fostering a successful relationship requires detailed research and effective communication.

Currently, pharmaceutical manufacturers are said to waste $25 billion on supply chain inefficiencies. Technology offers a way to achieve transparency and results.

Supply chain risk monitoring is crucial for any company doing business today, and it doesn’t have to be expensive.

More life-sciences companies are starting to manage global suppliers holistically.

By working together and taking a QA-based approach, manufacturers and suppliers can reduce raw material testing requirements.

Click the title above to open the Pharmaceutical Technology 2017 Partnerships in Outsourcing Supplement in an interactive PDF format.