
Feedback is urged on USP's proposed revisions to the General Notices.

Feedback is urged on USP's proposed revisions to the General Notices.

FDA approvals renew the threat of competition from India and China.

FDA's long-awaited GMPs for supplements appear as food and drug safety concerns override lingering opposition.

By vetoing stem cell research funding, the President is vetoing potentially life-saving treatments.

Makers of temperature-sensitive products constantly seek to ensure proper conditions during shipping and storage.
The "Solo-VPE" device from C Technologies (Bridgewater, NJ) improves UV–vis measurements by matching the length of the light path to the sample. The unit's software lets users establish thresholds, and the machine adjusts the pathlength and wavelength accordingly. Mark Salerno, director of manufacturing at C Technologies, says the device "... works for you until it finds the measurement range you want it to be in." He adds that the unit expands the operational range of UV–vis instruments. It facilitates measurements from roughly 50 μm to more than 10 mm. Measurements can be adjusted in 10-μm increments.

Did you know that only one-third of prescribed pediatric drugs have been studied or labeled for pediatric use? The Best Pharmaceuticals for Children Act (BPCA) was enacted in 2002 to improve this statistic by providing companies with six months of additional marketing exclusivity if they conduct pediatric trials. The Government Accountability Office recently issued a report that examined data from studies conducted between 2002 and 2005 for drugs specified by the US Food and Drug Administration under BPCA.

A spate of drugs are scheduled to come off patent, offering vast potential and competition.

The book comprehensively reviews the basics of drug absorption and methods for delivery enhancement through various routes of administration.
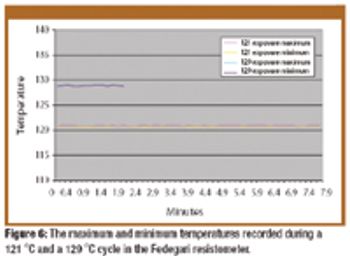
The authors examine advances in the design and the application of biological indicator evaluator resistometer vessels used to measure the resistance of bacterial spores in monitoring sterilization processes.

Production sometimes follows the law of supply and reprimand.

Cook, ABB, Tyco, and Mettler-Toledo Gain New Managment

Washington, DC (June 25)-More than five million US adults import prescription drugs from other countries, two million of them without an official prescription, according to a survey conducted by the Pharmaceutical Research and Manufacturers of America (PhRMA). Concerned about the number of counterfeit drugs entering the US, PhRMA launched the survey to determine who was importing prescription drugs and why.
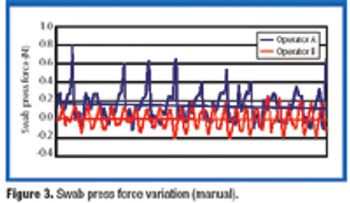
This article presents a study of an aseptic environmental monitoring system for surface contamination at critical areas using a robot.

USP is revising key documents to make them easier to use.

Industry and regulatory experts provide advice on inspection preparation and best practices.