
Implementing an electronic system to track out-of-specification results could help ensure compliance with current good manufacturing practices, but the system must be 21 CFR Part 11 compliant and easy to install, maintain, and use.

Implementing an electronic system to track out-of-specification results could help ensure compliance with current good manufacturing practices, but the system must be 21 CFR Part 11 compliant and easy to install, maintain, and use.
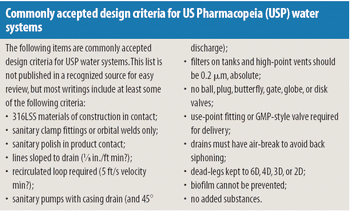
Water systems require a commonsense approach and good engineering rather than unquestioned acceptance.
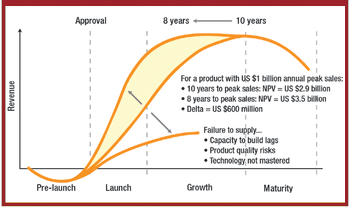
Companies must create a risk-based framework for developing and manufacturing drugs, and acquire the scientific knowledge and technological skills to create more complex products.

A contract manufacturing organization must clearly identify the goals of a project, establish a plan, and then effectively execute accordingly.