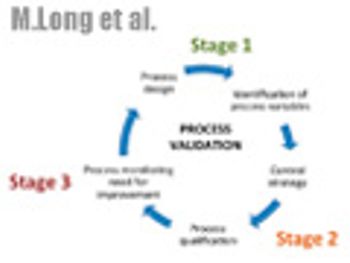
The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.

The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.

Excipients are the hidden champions of drug development-no API works consistently without the right excipient. Pharmaceutical excipients, however, require stringent quality management. This article discusses how the supplier of pharmaceutical raw materials should take a central role in ensuring excipient quality.

As contract manufacturers and fine-chemical suppliers gather for CPhI/ICSE, effective strategies for technology differentiation are key in an increasingly competitive environment.

A Q&A with Joe Cascone, director of potent pharmaceutical development at Metrics, moderated by Patricia Van Arnum. Discussion of the key considerations made in facility design, equipment selection, and operations to achieve desired levels of containment.

Clarifying GMPs for excipients used as actives.

A Q&A with Brian Johnson, senior director of supply chain security at Pfizer, moderated by Patricia Van Arnum. Part of a special Ingredients issue.
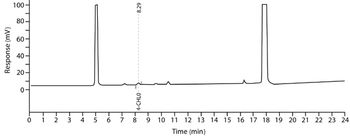
The authors provide an overview of methods for the quantitative determination of genotoxic impurities (GTIs) in active pharmaceutical ingredients.
A perspective from Pfizer on the lessons from small-molecule manufacturing that can be applied to biomanufacturing.

The author examines sample-preparations methods used in inductively coupled plasma–optimal emission spectroscopy for four test metals.