
Stakeholders work to meet deadlines, maximize benefits, and close gaps.

Stakeholders work to meet deadlines, maximize benefits, and close gaps.

Rapid scale-up to billions of doses requires collaborative, all-out efforts by innovators, their manufacturing partners, and the entire supply chain.

Research suggests that intranasal nanosuspensions offer a promising way to formulate and deliver drugs that are poorly soluble in water to patients with chronic conditions.

Risk-based decision-making is impacting all aspects of manufacturing quality from raw material supply to facility inspections.

Tight development timelines and accelerated approval pathways favor simple, cost-effective capsule formulations.
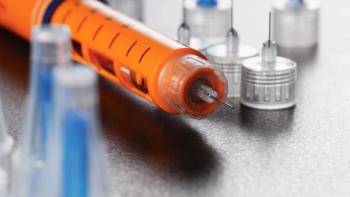
A device manufacturing process must be carefully designed in the early stages of development to ensure success in commercial manufacturing.

The general principle of lyophilization has hardly changed, but significant advances have occurred in process and product attribute understanding.

Despite pharmacovigilance legislation being in place for nearly a decade, many companies are still struggling to fulfill obligations.

Processes, people, and tools are needed to comply with the pharmacopoeia and approved drug product registrations.

Method-transfer kits help simplify analytical method transfer for global site certifications.

A new approach to testing water content in biologics is needed that will give a more accurate determination of actual water content in the biologic.

Regulators strive to review flood of advanced treatments while also vetting COVID-19 vaccines.

The 2020–2025 EMRN strategy will be regularly reviewed over the coming five years to accommodate science and technology advances.

AstraZeneca has potentially taken poll position in the race to develop a novel coronavirus vaccine, but will AZD1222 be ready in 2020?

Amid high expectations for a vaccine, bio/pharma readies capacity, weighs pressures.

An assessment can identify the critical systems and the gaps in compliance based on intended use, says Siegfried Schmitt, vice president technical, Parexel.

Pall has introduced Acrodisc syringe filters with universal water-wettable polytetrafluoroethylene (wwPTFE) membranes for analytical instrument applications.

MegaShear from Ross is a patented ultra-high shear rotor/stator mixer for high-throughput emulsification, dispersion, and homogenization.

GEA has expanded, integrated, and consolidated its ConsiGma continuous oral solid dosage equipment, allowing fully configurable systems that can be connected to new or existing facilities, including third-party equipment.

The Rosemount 550PT Pressure Transmitter from Emerson is designed for single-use bioprocessing applications to enhance processes while preserving product integrity.