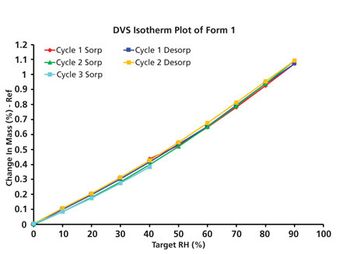
The author provides a direction for identifying genotoxic impurities early in the drug development process, regulating genotoxic impurities at acceptable levels in the API or drug product, and avoiding negative product regulation late in the development and/or marketing process, including expensive recalls.