
The numbers of new molecular entities approved in 2019 are close to or exceed FDA’s performance in most previous years.

The numbers of new molecular entities approved in 2019 are close to or exceed FDA’s performance in most previous years.

While labor and tariff reforms in the revised North American trade agreement may have more visible impacts on the United States economy, the final document levels a major blow to exclusivity and patent protections important to innovator biotech and pharma companies.

FDA has an official new leader, following a Senate vote to confirm Stephen Hahn as the agency’s next commissioner.
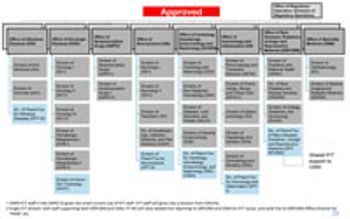
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.

Developers of biosimilars have become dismayed with difficulties in gaining acceptance and reimbursement from the US healthcare system.

A recent report released by an FDA task force highlights the financial, manufacturing, and policy issues underlying the drug shortages of important prescription medicines in the United States.

After months of planning and explaining, Janet Woodcock, director of the Center for Drug Evaluation and Research (CDER), finally gained approval for broad changes in its process and procedures for evaluating and approving new drugs.

Time is running out on Ned Sharpless’ term as FDA acting commissioner, generating much talk as the administration shows interest in naming a new head for the agency.

The multitude of health reform measures emerging on Capitol Hill has accelerated lobbying action across the board, along with campaign donations to candidates from all sides.

FDA, Health and Human Services, and the Trump Administration back cheaper foreign drugs to cut pharma costs.

The US Senate and the US Pharmacopeial Convention debate the necessity of monographs for biologics.

Congress is weighing in on drug pricing with a range of measures that differ in style and substance.

FDA is supporting the development and marketing of OTC therapies, including CBD products, by proposing new rules and an expanded review office and promoting digital technologies.

FDA is examining and updating its programs for overseeing global operations and international affairs.

CDER Director Janet Woodcock is finalizing this more streamlined approach process for evaluating new drugs to handle the surge in drug application submissions.

Sharpless will seek further solutions to the opioid crisis and work to reduce cigarette use in adults and kids.

Norman (Ned) Sharpless, director of the National Cancer Institute at the National Institutes of Health, will become FDA acting commissioner.

The FDA planned budget features added funds allotted to improved oversight of drugs, biologics, and medical devices.

Gottlieb’s tenure included record new drug approvals and steps taken to curb opioid abuse.

The agency clarified the process for development programs for regenerative medicine therapies.

FDA came away with the largest boost in its annual budget in a decade as part of the final spending package approved by Congress this week.

New HHS proposal would remove the current safe harbor protection from the federal Anti-Kickback Statute for rebates or discounts provided to Medicare Part D drug plans and certain state Medicaid programs.

FDA staffers will be hard-pressed to process and review the wave of new drug application submissions in pre-established timeframes for action.

Industry investment and regulatory support combined to move many important new medicines to market.

The proposal from the Centers for Medicare and Medicaid Services (CMS) proposes to give plans more flexibility to limit coverage of certain drugs.

Despite ongoing efforts to address the problem, FDA now sees a rise in active shortages and in the duration of supply problems.

Moving forward with gene therapy development requires a “quantum leap” in manufacturing capabilities.

Leaders of the two parties are open to challenging the status quo on drug costs and spending.

A new proposal aims to reduce reimbursement for medicines administered to seniors under the Part B benefit to an amount pegged to the average price paid in foreign industrial nations.