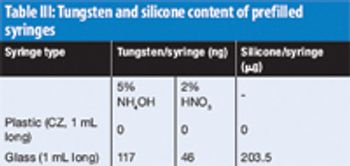
The authors investigate the effect of low pH and ionic strength on aggregation using turbidity measurements and size-exclusion–high-performance liquid chromatography.

The authors investigate the effect of low pH and ionic strength on aggregation using turbidity measurements and size-exclusion–high-performance liquid chromatography.

View presentations from the 2011 winners of AAPS Scientific Awards.

When I run a small production batch of a particular formulation with the same tablet press used to develop it, I get compressibility and disintegration issues. What am I doing wrong?

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.
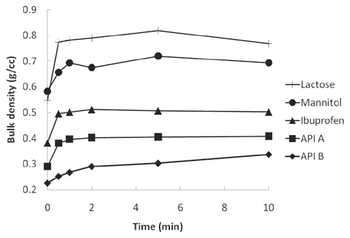
The authors experiment with a resonant acoustic mixer as a method for dry powder coating.

Average deal size and amounts invested increased in the second quarter, but totals trail funding of last year.

AAPS Global Health Focus Group's Kishor M. Wasan discusses new initiatives.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.

Could oral absorption-enhancing technologies change the shape of protein delivery?

The authors describe the concept of the limiting discriminatory the limiting discriminatory threshold (LDT) as an objective means of evaluating the inherent quality requirement of a large-sample content-uniformity test.

A Q&A with Rao Tatapudy, vice-president of scientific affairs at Catalent, on recent industry trends.

New product reviews for October 2011 focusing on analytical instrumentation.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

Where are the new excipients, the new solubilisers and sustained release excipients?

Internal and external Web-based communities are changing how pharma companies can innovate.
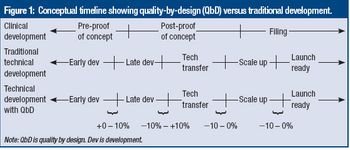
Directors from FDA's Center for Drug Evaluation and Research summarize findings in an FDA-commissioned report on QbD and propose actions the agency can take to encourage full-scale QbD implementation.

The EU debt crisis portends of possible negative repercussions for the dose CMO industry.

The author offers perspectives on ways in which pharmaceutical companies and other stakeholders in the supply chain can confront the threat of counterfeit products, cargo theft, illegal diversion, and economically motivated adulteration.

Might European officials reverse their position on acceptable production methods?

Reauthorization of pediatric exclusivity provisions looms in 2012 and debates begin anew.

International Federation of Pharmaceutical Manufacturers and Associations takes global action to improve public health.

Researchers at MIT and Harvard University report on new methods for producing microscale hydrogels.

Biocatalysis, chemocatalysis, and other chiral technologies continue to attract the investment dollars of CMOs and fine-chemical companies.

Direct dosing APIs during R&D studies can reduce the overall testing time of a drug candidate by allowing for a greater throughput of compounds through the R&D department.

The increasing cost of crucial manufacturing input factors, such as energy and raw materials, has been a severe threat to several companies.