In this technical forum, experts describe different methods of rapid microbial testing and their applications.

In this technical forum, experts describe different methods of rapid microbial testing and their applications.

If a product does not have its own antimicrobial properties, then a preservative must be used to ensure microbiological safety.

There are various theories about how to scale up a solid dosage coating operation in a pan coater. This article provides a basic process understanding and scale-up theory based on first principles.
In Part III of a three-part article, the authors examine various degradation routes of APIs, impurities arising from interactions in formulations, metabolite impurities, various analytical methods to measure impuritie, and ways to control impurities.

Critical issues that should be considered when scaling up a hot-melt extrusion process.

With financing constrained, biotechnology firms must find ways to sustain innovation.

Contract API manufacturers and fine-chemical producers roll out capacity and service expansions.

Excipient manufacturers expand production capacity and partner to broaden their offerings.
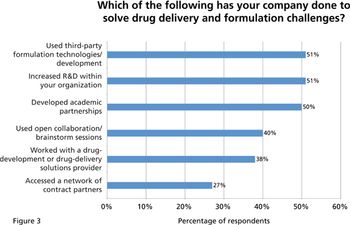
A recent survey examines the industry's views on the chief challenges and technologies in drug delivery and formulation development.

Charles E. Seeney tells us about the possibilities of nanotechnology and magnetism, and how a novel approach could improve localised drug delivery.

In our tablet coating process, we are losing up to 15% of our coating solution in each processing run. What can we do to prevent this problem in order to reduce waste and increase our cost efficiency?

On Mar. 7, 2012, GE Healthcare announced an agreement to acquire Xcellerex, a supplier of manufacturing technologies for the biopharmaceutical industry, for an undisclosed amount.

Nanosupensions are among the ways formulation scientists seek to address the problem of solubility.

Regulatory bodies, standard-setting organizations, and industry seek to tackle the problem of counterfeit drugs and securing the flow of pharma ingredients.

The divide between innovation and conflict of interest in medical research is not so clear.

Genotoxic impurities and how to identify them and control for them have been a concern for several years in the pharmaceutical manufacturing industry. Pharmaceutical Technology spoke with Bo Shen, PhD, principal scientist at Amgen and chair of the AAPS Pharmaceutical Trace Impurities Focus Group, to gain insight on key challenges.

In Part II of a three-part article, the authors examine impurities from chiral molecules, polymorphic contaminants, and genotoxic impurities.

Wirelessly controlled microchips may offer an alternative to injection-based drug delivery

The evolving bio/pharmaceutical business model poses risk for CMOs.

The author reviews significant changes to GMP for excipients in the forthcoming American National Standard, including a risk-based approach to excipient manufacture, why new requirements were proposed, and their potential impact to excipient manufacturers.

This article provides guidance for industry on how to comply with the pending American National Standard on excipient GMP, with a focus on risk assessment.
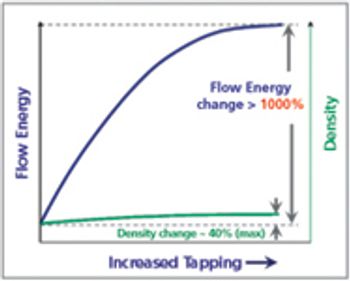
This article considers the different conditions to which the powder is subjected in the tableting process, and discusses which powder properties should be measured to accurately reflect likely powder behavior in the process.
Pharmaceutical companies, equipment providers, contract-service providers, and excipient manufacturers apply various approaches for improving solubility. The article examines some recent developments.

Taste-masking is an important consideration to ensure patient compliance.

Experts in solid dosage discuss the formulation and manufacture of multilayer tablets.